
Please email or share this article!

Mentos and Coke Experiment – How to Do It! [Full Guide]
Have you ever taken a can of soda, shook it up, and given it to your friend?
What happens?
Well, it’s probably not pretty. And you may not have a friend for a day or two until they forgive you.
But making soda explode is fun. And there is a way to make it really go boom if you have a few pennies and a bit of time on your hands.
Note – be sure to only try this experiment with a responsible adult!

It really only takes a few minutes to setup the mentos and coke volcano experiment. And it’s a great way to learn about chemical reactions.
It’s also a lot less work than your classic paper mache volcano. So, if you want some quick and easy fun, get some paper towels because we’re about to make a sweet mess.
What Will I Need For The Mentos And Coke Volcano?
There really aren’t that many supplies you need to make a mentos and coke volcano.
But here’s the list:
An outdoor area with no ceiling or roof
One roll of Mentos candies
A two-liter bottle of diet soda (diet soda makes for a much better reaction, but you can use regular soda if you like. It just won’t be nearly as awesome.)
A tube the width of the Mentos . It needs to be wide enough to use as loader for the Mentos
An index card (picture below)

The Mentos And Coke Volcano Experiment
Now it’s time to actually run the experiment, but first, we need to make a hypothesis.
The Hypothesis
The scientific method is an important way scientists make observations and come to conclusions.
Part of the scientific method is making a prediction called a hypothesis .
Write down what you think will happen when placing the Mentos in the soda bottles.
Do a little bit of research about the ingredients of Mentos and soda.
This will help you make an informed guess as to what will happen.

Now You Test The Hypothesis
In an experiment, you have two groups: an experimental group, and a control group.
Open the soda bottle, set it down and write down what you observe about it.
This will count as your control group. It’s what happens when you put nothing in the soda.

Now take the Mentos in your tube loader.
Put the index card on top of the tube loader and turn the tube upside down.
The candy should not fall out.
Be ready. The reaction happens fast, so don’t have your face over the bottle.
Place the index card and candies over the mouth of the bottle. Make sure the candies are in line with the mouth of the bottle.
You want the candies going in the bottle and not falling over the side.
Now remove the index card and let candies fall in and step away from the Mentos and coke volcano.

Write down what happened when you dropped the Mentos in the coke.
Did what you hypothesize happen? Compare your notes on the experiment to the control group.
Let us know what you observed in your science experiment!
FREQUENTLY ASKED QUESTION
1. can i use any type of mentos candy for the experiment.
Yes, you can use any type of Mentos candy for the experiment. The most commonly used Mentos candies are the original mint-flavored ones, but you can also use fruit-flavored or other varieties. The key factor is the rough surface of the Mentos candy, which helps to create nucleation sites for the carbon dioxide bubbles in the Coke. This happens because Coke contains dissolved carbon dioxide gas.
2. What happens if I use diet Coke instead of regular Coke?
If you use cold diet Coke instead of regular Coke in the Mentos experiment, you can still expect an explosive reaction. However, the reaction may not be as vigorous as with regular Coke. Diet Coke contains artificial sweeteners like aspartame, which may slightly affect the reaction. Nonetheless, the combination of Mentos and diet Coke can still produce a notable geyser, so it’s worth giving it a try. SO it will be the mentos geyser experiment.
3. Is the Mentos and Coke experiment suitable for children to try at home?
The Coke and Mentos experiment can be a fun and engaging activity for children to try at home. However, ensuring proper adult supervision and following safety precautions is important. Conducting the mentos experiment outdoors or in a well-ventilated area is recommended to avoid any potential mess or accidental spills. Additionally, remind children not to consume the Coke or Mentos mixture, as it is unsafe for ingestion. By taking these precautions, the Coke and Mentos experiment can provide children an educational and entertaining experience.
Leave a Comment
Get Your ALL ACCESS Shop Pass here →

Erupting Mentos and Coke Experiment
Love fizzing and exploding experiments? YES!! Well, here’s another one the kids are sure to love! All you need are Mentos and Coke. Put the scientific method into practice with two easy-to-set-up Mentos science experiments. Record your results with a video camera so you can enjoy seeing the exploding fun up close (and over and over again)! Learn all about the Mentos and Coke reaction!

Grab Some Mentos and Coke
Our Mentos and soda experiment is a fun example of a physical reaction. Read on to learn more about how this Mentos and Coke reaction works.
We love fizzing experiments and have been exploring science for kindergarten, preschool, and early elementary for over 8 years now. Make sure to check out our collection of simple science experiments for kids.
Our science experiments are designed with you, the parent or teacher, in mind! Easy to set up, and quick to do, most activities will take only 15 to 30 minutes to complete and are heaps of fun! Plus, our supplies lists usually contain only free or cheap materials you can source from home!
Grab a packet of Mentos and some Coke as well as assorted soda flavors, and find out what happens when you mix them together! Do this activity outside to make clean-up a breeze. Make sure to put it on a level surface so the cups don’t tip over.
ALSO CHECK OUT: Pop Rocks and Soda
NOTE: This experiment is a less-mess version and more hands-on for younger kids. See our Mentos Geyser version for a bigger eruption!
Coke and Mentos Reaction
You might be surprised to know that the Mentos and Coke reaction is an example of a physical change ! It’s not a chemical reaction like how baking soda reacts with vinegar and a new substance, forming carbon dioxide. So how does it work?
Inside the Coke or soda, there is dissolved carbon dioxide gas, making the soda taste fizzy when you drink it. Usually, you can find these gas bubbles coming out of the soda on the sides of the bottle, which is why it becomes flat after a while.
Adding Mentos speeds up this process because more bubbles form on the Mentos’s surface than on the bottle’s side, pushing the liquid up. This is an example of a change of state of matter . The carbon dioxide dissolved in the Coke moves to a gaseous state.
In the first experiment, if the size of the Mentos is the same, you will notice no difference in the amount of foam produced. However, when you make the pieces of Mentos smaller it will cause more bubbles to form and speed up the physical reaction. Give it a go!
In the second experiment, when you test out Mentos with different sodas, the soda that produces the most foam will likely have the most dissolved carbon dioxide or be the fizziest. Let’s find out!

Mentos and Coke Experiment #1
Do Coke and Mentos work with fruit Mentos? You can do this experiment with any Mentos! This first experiment uses the same soda to test which variety of candy creates the most foam. Learn more about independent and dependent variables.
TIP: Mentos and coke at room temperature generally produce the best results.
- 1 sleeve Mentos Chewy Mint candy
- 1 sleeve Mentos Fruity candy
- 2 (16.9 to 20 ounce) bottles of soda (diet sodas tend to work the best.)
- Video camera or smartphone with video (for replay)
HOW TO SET UP MENTOS AND SODA EXPERIMENT #1
STEP 1. To analyze the results, set up a video camera or smartphone with video capabilities to capture the experiment.
STEP 2. Prepare the candy by removing the different types from their sleeve and placing in separate cups.

STEP 3. Pour equal amounts of the same soda into two other cups.

STEP 4. Make sure the camera is recording, and drop the candy into the soda simultaneously. One variety of candy goes into one cup of soda, and the other variety goes into the other cup of soda.

STEP 5. Analyze to see which variety of Mentos creates the most foam. Was there any difference?
Mentos and Coke Experiment #2
What type of Coke reacts best with Mentos? In this second experiment, use the same variety of Mentos and instead test to find out which kind of soda creates the most foam.
- 3 sleeves Mentos Chewy Mint candy OR Mentos Fruity candy
- 3 (16.9 to 20 ounce) soda bottles in different varieties (diet sodas tend to work the best.)
HOW TO SET UP COKE AND MENTOS EXPERIMENT
STEP 2. Choose one variety of Mentos candy to use for the experiment. Prepare the candy by removing it from the sleeve and placing one sleeve of candy into each cup.

STEP 3. Pour equal amounts of the different sodas into cups.

STEP 4. Simultaneously, drop the candy into the soda.
STEP 5. Look at the video and analyze which variety of soda creates the most foam.

Expand the Mentos and Coke Experiments
- Test cups, bottles, and vases of different shapes (wide at the bottom but narrow at the top, cylindrical, or directly in the soda bottles) to test whether the width of the cup makes a difference in how high the foam will shoot.
- Design unique ways for dropping the candy into the soda. For instance, create a tube that fits around the mouth of the soda bottle. Cut a slit into the tub that runs ¾ across the width of the tube. Slide an index card into the cut slit. Pour the candy into the tube. Remove the index card when you are ready to release the candy into the soda.
- Add different ingredients to the soda to test whether the amount of foam changes. For instance, we have tested adding food coloring, dish soap, and/or vinegar to the soda while adding baking soda to the cup with the candy.
TIP: Want to try the more traditional Mentos and Coke Rocket, see it here!

Turn It Into A Mentos and Coke Science Fair Project
Science projects are an excellent tool for older kiddos to show what they know about science! Plus, they can be used in various environments, including classrooms, homeschool, and groups.
Kids can take everything they have learned about using the scientific method , stating a hypothesis, choosing variables , and analyzing and presenting data.
Want to turn this Coke and Mentos experiment into a cool science project? Check out these helpful resources below.
- Easy Science Fair Projects
- Science Project Tips From A Teacher
- Science Fair Board Ideas

More Helpful Science Resources
Here are a few resources to help you introduce science more effectively to your kiddos or students and feel confident when presenting materials. You’ll find helpful free printables throughout.
- Scientific Method For Kids
- Best Science Practices (as it relates to the scientific method)
- Science Vocabulary
- 8 Science Books for Kids
- All About Scientists
- Science Supplies List
- Science Tools for Kids
More Fun Science Experiments to Try
- Baking Soda and Vinegar Volcano
- Lava Lamp Experiment
- Soda Balloon Experiment
- Pop Rocks and Soda
- Magic Milk Experiment
- Egg In Vinegar Experiment

Printable Science Projects For Kids
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.

- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School
The Infamous Coke and Mentos Experiment
September 11, 2014 By Emma Vanstone 1 Comment
The science experiments my children talk about for months afterwards are generally the messy ones, like our splatter patterns , glow in the dark oobleck , baking soda experiments and the well known coke and mento experiment .
If you try this classic chemis t ry experiment definitely do it outside as it’s VERY messy and sticky. Sometimes you’ll see it called a coke and mento geyse r, as the eruption looks like a geyser!

Coke and Mentos Experiment
You’ll need:.
Coke or other fizzy soda
Instructions
We dropped two Mentos into a bottle of normal Cola and Diet Cola. I used the cheapest brands available in our local supermarket.

Once you drop the Mentos into the coke, stand back as it’s VERY explosive. The trick is to drop the mento in as fast as you can. If too much of the fizz escapes before you add the mento the reaction won’t be as good.

What happens when Coke and Mentos mix?
There are several theories, but it’s thought that the many small pores on the surface of the mento speed up the release of carbon dioxide (CO 2 ) gas from the soda as they give a larger surface area for the reaction to occur over, causing foam to erupt at a super fast rate.
Which soda works best with Mentos?
Any fizzy drink will produce a similar effect, but diet drinks seem to work best, as we found in our investigation. This is most likely due to the particular chemicals in diet drinks.
The reaction isn’t a chemical reaction but a physical reaction! The molecules haven’t been chemically changed, just re-arranged!
See Steve Spangler for a much more thorough explanation of this very cool experiment .
Does the number of mentos affect the height?
More Mentos candies should mean a better explosion, but there is a limit to how many will actually make a difference. We found 7 to be the maximum number we could drop in at once.
More Coke and Mentos Eruption Ideas
Investigate to find out if the type of fizzy drink matters. Does diet soda make a taller geyser?
Try lots of different sodas and diet sodas.
Test fruit-flavoured Mentos instead of mint flavoured.
Find out if the number of mentos affects the height of the geyser.
Investigate to discover what would happen if you left the top off the Cola for a few minutes before adding the Mento.
Use the reaction to power something? Maybe a LEGO car?
Design a device to drop several mentos into the bottle at the same time. Can you find out what the optimum number of mentos for a 2-litre bottle of soda is?

Last Updated on April 9, 2024 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
December 17, 2019 at 7:20 pm
It will also work better the warmer the soda is
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Coke and Mentos Experiment – Easy STEM for Kids
Categories Science , STEM
The Coke and Mentos Experiment is an easy science experiment to do with kids of all ages. Even toddlers and preschoolers will shriek with delight as they watch this chemical reaction take place.

Disclosure: Adult supervision is required for all activities at all times.
Table of Contents
- More STEM activities to try
- Materials needed
- Watch the video
Instructions
- Experiment ideas
- How does it work?
STEM Activities for Kids
Help your toddlers and preschoolers become the next new scientist or engineer with these fun STEM activities. These are great for getting little learners involved in STEM through hands-on play.
- How to Make A Pom Pom Shooter
- Floating City – STEM activity for Kids
- Building Shapes – STEM for Kids
- Magnet Exploration
For more ideas check out 34 STEM Experiments for Toddlers .
Materials Needed
- A large bottle of coke
- A packet of mentos
- A plastic tub
- Hot glue gun and hot glue sticks (optional)
Watch the Video

Coke and Mentos Experiment Instructions
1: use the hot glue gun to glue 6 mentos together..

You can always use more or less mentos if you need to, the number of mentos will depend on how large your bottle of coke is.
Gluing the mentos together is an optional step, but one that I find invaluable when doing this experiment with toddlers and preschoolers. Because the reaction happens instantly, it’s hard to drop all of the mentos in before the coke starts to come out of the bottle.
By creating a mentos tower, the kids can drop all of the mentos in at once.
2. Place your bottle of coke in a large tub.

Once again the tub is optional and is only used to help clean up afterwards. If you prefer, you can do this experiment outside on the grass. Just make sure that your outdoor area has a flat surface you can use. If the bottle of soda falls over before you’ve dropped in the mentos candy, you’re going to be disappointed.
3. Drop your mentos tower into the bottle of coke, stand back and enjoy.


Different Ways to turn this into an Experiment
If you are doing this experiment with older children, you can use the scientific method to see which conditions affect the height of the geyser.
Idea 1. Change the Temperature.
Does cold, room temperature, or warm soda affect the amazing eruption? Place a bottle in the fridge, one in the sun and one at room temperature to find out if any of these temperatures create a large eruption.
Idea 2. How many mentos are needed in a coke and mentos experiment?
You’ll need at least 7 – 8 mentos for this activity. However you can always experiment with a different number of mentos to find out. Set up several soda bottles and then place a different number of mentos in each bottle and measure the fountain height to see which worked best.
Idea 3. Which soda works best with mentos?
You can use any type of soda pop you like for this experiment. While it’s believed that Diet Coke has the most impressive results, the truth is that there isn’t much difference in the height of the eruption between diet and regular soda.
The main difference is when cleaning up. Regular coke becomes sticky because of the high sugar content. While diet soda instead contains artificial sweeteners. Cleaning up the mess is another reason why outdoors is the perfect place to do this soda experiment.
However, you can always experiment with different types of soda and see what happens. Mountain dew, classic diet coke, orange soda are all options you can try.
How does the coke and mentos experiment work? Coke and Mentos Explained
Coke, and other carbonated beverages are filled with dissolved carbon dioxide gas. This gas has formed bonds with the water in the soda.
In order for the mentos reaction to take place, the gas needs to break the bonds with the water and interact with the rest of the carbon dioxide gas in the coke. When carbon dioxide interacts with itself it forms gas bubbles. That is why we can see bubbles form when we pour a glass of coke to drink or shake the bottle up.
When you drop a tower of mentos into the bottle, they break the bonds that hold the carbon dioxide gas and water together. Because the mentos tower sinks to the bottom of the bottle, instead of floating, this means that the whole bottle will undergo this reaction at once.
These bubbles come rushing out at great speed and because the coke bottle has a small opening, the bubbles rush high into the air. Creating an eruption effect.
While toddlers and preschoolers aren’t going to understand why this chemical reaction takes place, it’s still cool to learn as an adult. I love Steve Spangler’s explanation of the chemistry involved in this STEM idea.
Are you going to try the Coke and Mentos Experiment with your kids? Don’t forget to Pin the idea for later.

Coke and Mentos Experiment

The Coke and Mentos Experiment is an easy science experiment to do with kids of all ages. Even toddlers and preschoolers will shriek with delight as they watch this chemical reaction take place.
- Use the hot glue gun to glue 7 - 8 mentos together. This is optional, it just helps to add all of the mentos into the bottle at the same time.
- Place your bottle of coke in a large tub. This is optional, it just makes cleaning up more easy.
- Drop your mentos tower into the bottle.
- Stand back and enjoy.
If you are doing this STEM activity with older kids, you can experiment with a few different ideas to introduce them to the scientific method.
- Change the temperature of the coke. Experiment to see if cold, warm or room temperature coke changes the reaction.
- Change the number of mentos.
- Experiment to see which soda works best. Coke, diet coke or any other kind of soda. Which one has the best results?
More Play Based Learning for Kids

Share this:
- Click to share on Facebook (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Experiments
- Secret Science of Stuff
- Science ABCs
- Mentos and Diet Coke!
- You are here:
- American Chemical Society
- Adventures in Chemistry
Have you ever noticed that when you put a straw in soda pop, the straw gets a lot of bubbles on it? Why does that happen? And will it happen if you put other stuff in soda pop?

1. Very slowly and carefully, open a new bottle of colorless soda.
2. Tilt the cup and slowly pour the soda down the inside of the cup to make as few bubbles as possible.

3. Place a straw in the soda and look at the straw from the side.
4. Take the straw out of the soda and put a pipe cleaner in. Look from the side to see if bubbles also form on the pipe cleaner.

5. Now take the pipe cleaner out and place a Mento in the soda. Watch the Mento from the side to see what happens.
What to expect
Bubbles will form on the straw and very quickly and completely cover the pipe cleaner.
What's happening in there?
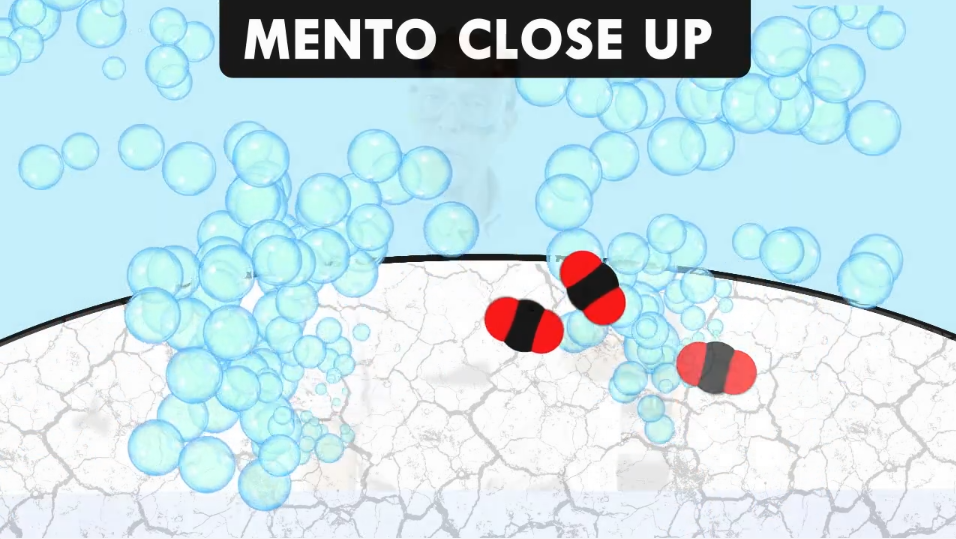
Why do the bubbles form on the different things you put in the soda?
The bubbles are made of a gas called carbon dioxide. The soda company puts carbon dioxide in the soda to make the soda fizzy.
Also, the things you put in the soda aren’t really as smooth as they look with just your eyes. If you could look at the straw, pipe cleaner, and Mento with a super-strong microscope you would see that they have tiny dents, scratches, and bumps on them.
The carbon dioxide molecules collect on these places and form bubbles which rise to the surface.
Make a Mentos-and-Soda Fountain!
There is a pretty cool thing you can do with a bottle of soda pop and a packet of Mentos. Let’s try it!
First, make a tube for the Mentos.

1. Cut a piece of paper so that it is as wide as a roll of Mentos.
2. Wrap the paper around the pack of Mentos to make a tube. Use masking tape to tape the tube closed. Remove the pack of Mentos from the tube.
3. Close off one end of the tube by cutting a little circle or square of paper and taping it to one end of the tube.
4. Open the pack of Mentos and place all of them in the tube.
Now, make a Mentos-and-soda fountain!

1. Slowly and carefully open a new bottle of Diet Coke.
2. Place it on a flat area outside where it is OK to get wet with soda.
3. Put the open end of your tube of Mentos on the card and place it directly over the opening of the soda bottle.
4. When you are ready, remove the card and let all the Mentos drop into the soda at once and quickly move out of the way.
Bubbles and soda will quickly shoot out of the bottle in a high fountain.
The carbon dioxide molecules attach to the surfaces of the Mentos like they did in the cup of soda. All those Mentos in a lot of soda make a lot of bubbles that rise to the surface and push the soda out in a big woosh!

- Resources for Parents and Teachers
- Terms of Use
- Accessibility
Copyright © 2024 American Chemical Society
- Grades 6-12
- School Leaders
Don't Miss Today's Holiday Giveaway!🎁
Every product is independently selected by our team of teacher-reviewers and editors. Things you buy through our links may earn us a commission.
Mentos and Coke Experiment: How-To Plus Free Worksheet
This explosive experiment teaches kids about physical reactions.

Adding Mentos candy to Coke is the stuff of legend. Every kid has heard the rumors about the explosive combination that results, but how many have actually tried it? While science teachers have been performing this experiment for years, it was first popularized in September of 2005 thanks to a viral video from Steve Spangler Science . The several-foot-high geyser that shoots from the soda bottle is a fun and awe-inspiring hands-on activity that any scientist in the making can perform. Be forewarned though: You’ll probably want to perform this experiment outside.
Read on to learn more about the Mentos and Coke experiment, and fill out the form on this page to grab your free recording sheet for the experiment.
How does the Mentos and Coke experiment work?
In this experiment, you drop Mentos mints into a 2-liter bottle of Coke. Make sure your bottle of soda is on a flat surface in a location where it is OK to make a mess. You then load the Mentos into your paper roll or geyser tube . Once the Mentos are dropped into the soda, they sink to the bottom, which causes the gas to expand and pushes the soda out of the bottle. This creates an exploding geyser effect.
What does the Mentos and Coke experiment teach?
Although you can’t see it, dissolved carbon dioxide is the invisible substance that makes soda bubbly and fizzy. As long as the soda remains in the bottle, the gas is kept in place through the pressurized conditions. When you shake a bottle of soda, some of that gas is released and the bubbles stick to nucleation sites or tiny defects on the inside of the container. If you open the shaken bottle, the bubbles will rapidly rise and push the liquid up and out of the bottle.
Aside from shaking the soda, another way to help the carbon dioxide escape is to drop an object into the bottle. Mentos are the perfect objects, since each candy has many little pits on its surface that serve as nucleation sites. Once the Mentos are dropped into the soda, the bubbles stick to those sites and quickly rise to the surface. The weight of the Mentos drives them to the bottom of the bottle. Then, the gas that is released by the Mentos forces the soda to shoot out of the bottle in a powerful geyser.
Is there a Mentos and Coke video?
This video shows how to do the Mentos and Coke experiment using just a few simple ingredients and supplies.
Materials Needed
To do the Mentos and Coke experiment, you will need:
- A roll or box of mint-flavored Mentos
- 2-liter bottle of Coca-Cola (aka Coke)
- Sheet of paper to roll into a tube OR pre-made geyser tube
Our free recording sheet is also helpful—fill out the form on this page to get it.
Mentos and Coke Experiment Steps
1. make a paper tube by taking a piece of paper and wrapping it around a roll of mentos, then taping it in place. pull the mentos out. alternatively, you can use a premade geyser tube available from amazon or other retailers..

2. If using a geyser tube, load the Mentos. If using a homemade paper roll, drop the Mentos into the roll while holding the bottom closed with your finger.

3. Placing a 2-liter bottle of Coke on a flat surface, remove the cap, and drop the Mentos into the open Coke bottle.

Grab our free Mentos and Coke experiment worksheet!
Fill out the form on this page to get your worksheet. The worksheet asks kids to guess the correct order of the steps in the experiment. Next, kids must make a prediction about what they think will happen. They can use the provided spaces to draw what happens before and after they add the Mentos. Did their predictions come true?
Additional Reflection Questions
- What happened when we added the Mentos to the Coke?
- What difference do you think the temperature of the Coke makes?
- What do you think would happen if we used different-flavored Mentos, like fruit?
- What do you think would happen if we used a different soda other than Coke?
- What do you think would happen if you use Diet Coke?
Can the Mentos and Coke experiment be used for a science fair?
Yes! If you want to do the Mentos and Coke experiment for a science fair, we recommend switching up some of the variables. For example: Does the temperature of the Coke matter? Does the brand of soda matter? Will generic soda produce the same results as the brand-name soda? What happens if you use fruit-flavored Mentos? What happens if you use Diet Coke instead of regular? Form a hypothesis about how changing the variables will impact the experiment. Good luck!
Looking for more experiment ideas? Check out our big list of experiment ideas here.
Plus, be sure to subscribe to our newsletters for more articles like this., you might also like.

76 Easy Science Experiments Using Materials You Already Have On Hand
Because science doesn't have to be complicated. Continue Reading
Copyright © 2024. All rights reserved. 5335 Gate Parkway, Jacksonville, FL 32256
June 14, 2012
Spurting Science: Erupting Diet Coke with Mentos
A carbonated challenge from Science Buddies
By Science Buddies
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Key concepts Chemistry Physics Materials science Carbonation Physical reactions Explosions Introduction Have you ever seen the Diet Coke and Mentos experiment that is all over the Internet and wondered what makes the reaction work? You might think that there is some ingredient in a Mentos candy that causes a chemical reaction with the soda pop, like the way baking soda reacts with vinegar. But the amazing eruption that takes place when Mentos are dropped into Diet Coke or other brands of diet soda pop is not a chemical reaction at all! Instead it is a physical reaction. That means that all of the pieces of the reaction are there, but that they are simply rearranged. It also means changing some factors may cause a larger or smaller physical reaction to take place. Background A carbonated beverage is packed full of dissolved carbon dioxide gas, which forms bonds with water. While the soda is in the bottle, the gas is kept in solution by the bottle's pressurized conditions. When you pour some soda into a glass, some gas escapes and forms foam, but most stays trapped by the surface tension of the water. But all those gas bubbles want to escape, making it no wonder that soda makes you burp! To create bubbles, the carbon dioxide needs to interact with itself, which means that the carbon dioxide's bonds with water in the Diet Coke must be broken. A Mentos candy can help with this. Although the candy may look smooth, if you looked at it under a microscope you'd see tiny bumps coating its entire surface. This rough surface allows the bonds between the carbon dioxide gas and the water to more easily break, helping to create carbon dioxide bubbles and cause the classic eruption. The speed at which the Mentos falls through the soda can affect how large the eruption is, and this can be tested by comparing whole with crushed Mentos, the latter of which are less dense. Materials • Wax paper • Cutting board • Knife • One roll of Mentos (at least eight candies) • Two index cards • Tape • Two two-liter bottles of Diet Coke • An outdoor area at least two meters from buildings • Eye protection (safety goggles or glasses) • Video camera with either a tripod or a helper to take the images (optional) Preparation • Place a piece of wax paper on top of the cutting board. On the wax paper, carefully use a knife to crush and cut four Mentos candies into many small pieces. An adult may help you cut up the candies. What does the inside of the candies look like? • Make a Mentos cartridge to hold the candies for you before you drop them into the Diet Coke bottle by rolling an index card into a tube, slightly larger than the diameter of a Mentos candy. Tape the tube together on the side. • Be sure to wear eye protection when putting the candies into the cola! • Wear clothes that you would not mind if they get splashed with a little soda pop—this activity can get a little messy! Procedure • Place a Diet Coke bottle in an outdoor area, at least two meters from any buildings or anything hanging above the area, such as eaves, overhangs or wires. Make sure that the bottle is on a level surface and stably standing straight. Why do you think all of this is important? • If you want to videotape the reactions, set up the video camera so that it has in its viewfinder the bottle and a height equivalent to at least the first story of a building. • Carefully remove the cap from the bottle and place the flat index card on top, covering the hole. • Add four whole Mentos candies to your cartridge, put on your eye protection, and start the video camera. • Place your full cartridge on top of the flat index card. Line up where the opening of the bottle is with the opening of your cartridge. Quickly pull out the flat index card, releasing the Mentos candies into the bottle. Then step back without tipping the bottle over or disturbing the reaction. • How quickly did the reaction start to happen, and how quickly did it stop? About how high did the eruption go? How much cola is left in the bottle? • When the bottle stops spouting, stop recording. • Remove the spent cola bottle and place a new full bottle in the same position, again making sure that it is level and stably standing straight. As with the first bottle, remove the cap and place the flat index card on top, covering the hole. • Add your four crushed Mentos candies to your cartridge, pouring them in from the wax paper. Put on your eye protection and start the video camera. • Like you did before, place your full cartridge on top of the flat index card, then line up where the opening of the bottle is with the opening of your cartridge. Quickly pull out the flat index card, releasing the crushed Mentos into the bottle, then step back without tipping the bottle over or disturbing the reaction. • How quickly did the reaction start to happen, and how quickly did it stop? How high did the eruption appear to go? How much liquid is left in the bottle? Is it more or less than the amount that was left when you used whole candies? • When the bottle stops spouting, stop recording. If you videotaped the reactions, you can watch your videos now. What do you notice from the videos? • Which reaction went higher, the whole or the crushed Mentos? • Extra : Find an exterior wall of a building with no windows and set a Diet Coke bottle at the base of the wall. Use a tape measure and blue painter's tape to mark off the height from the top of the bottle in meters. Then repeat this activity three times, with the bottle in front of the tape-marked wall, video taping it each time. When you review the recordings, use slow motion and pause the recording when the spout is at its maximum height. Using the tape marks in the background, estimate the height of the spout. Calculate the average height of the fountains for the whole and for the crushed Mentos . What is the difference in height of the eruptions? • Extra: What other factors affect the size of the Mentos and Diet Coke eruption? You can try testing different kinds of carbonated beverages, different kinds of candies with different shapes and textures or using other things to start the reaction, like rock salt, pennies or dice. Which beverages, candies or other things cause the largest and smallest fountains? Why do you think this is? • Extra: Do this activity again but instead of testing whole Mentos versus crushed, compare warm versus cold Diet Coke. Does temperature affect the eruption height? Observations and results Was the eruption higher when whole Mentos candies were used compared with crushed candies? Was less Diet Coke left in the bottle after the reaction with the whole candies compared with the crushed ones? In the Diet Coke bottle the Mentos candy provides a rough surface that allows the bonds between the carbon dioxide gas and water to break more easily, helping to create carbon dioxide bubbles. As the Mentos candy sinks in the bottle, the candy causes the production of more and more carbon dioxide bubbles, and the rising bubbles react with carbon dioxide that is still dissolved in the soda to cause more carbon dioxide to be freed and create even more bubbles, resulting in the eruption. Because Mentos candies are rather dense, they sink rapidly through the liquid, causing a fast, large eruption. The crushed Mentos candies, however, are not as dense as the whole ones, which causes them to sink more slowly, creating a relatively small cola fountain, which should also leave more liquid in the bottle than the larger eruption with whole Mentos candies did. Cleanup Hose off any part of a building that was splashed with Diet Coke. If you try this project with regular Coke, the eruption should still happen but its sugary content may make cleaning more difficult. More to explore Physicists Explain Mentos–Soda Spray from Scientific American Science of Mentos–Diet Coke explosions explained from New Scientist The Science of Coke and Mentos from EepyBird.com Why do Mentos mints foam when you drop them into soda pop? from General Chemistry Online Coke® & Mentos®—Nucleation Goes Nuclear! from Science Buddies
This activity brought to you in partnership with Science Buddies

Diet Coke and Mentos Experiment
- Pinterest 19
Have you seen the Diet Coke and Mentos experiment that’s all over YouTube and social media? My son saw a video and has been eager to try it, so we finally did a couple of weeks ago. Here are some pictures and tips from our little eruption experiment so you can try it, too!

Diet Coke and Mentos Eruption
My son saw one of his favorite YouTubers doing this experiment a while back, and he’s been asking to try it ever since. When I finally got my brain together enough to add both ingredients to my shopping list, he was over-the-moon excited to see it when he got home from school.
Unfortunately, my store only carried the 12-ounce bottles of diet coke rather than the larger 16 ounce bottles. I suppose a 2-liter would have been better, but I thought the 12-ounce bottles would be better for smaller hands. We had a lovely little tiny eruption, but if you want a huge geyser, you definitely need a larger bottle of soda AND the ability to drop the Mentos candies in much quicker than my son did.
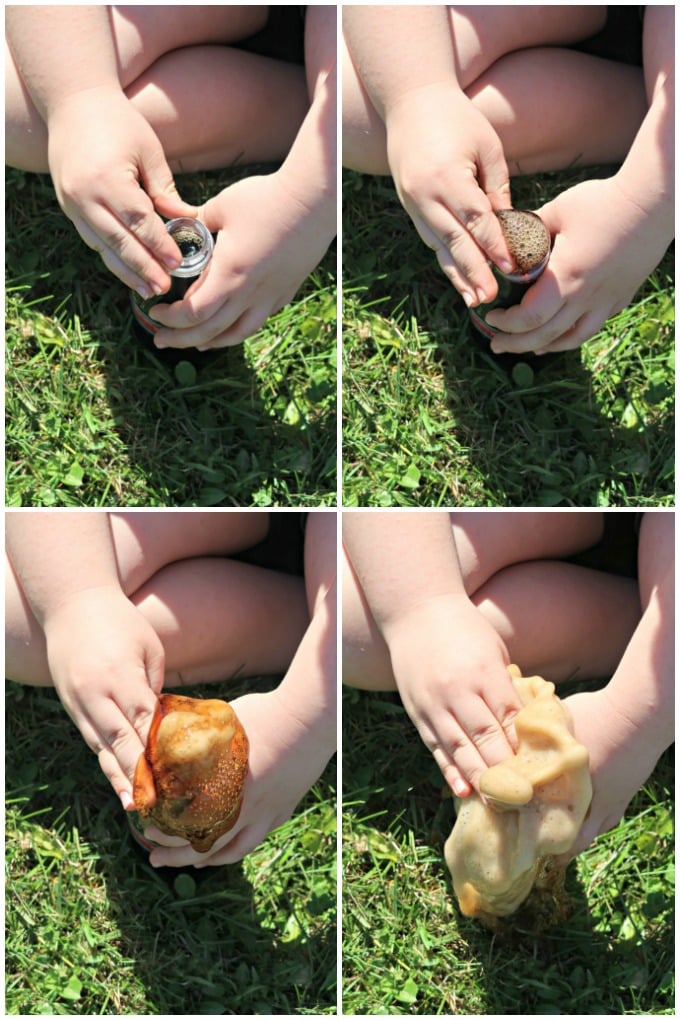
Even though we didn’t have a big geyser, he loved the experiment. We repeated it five times, and he kept sticking his hands over the bottle each time. This is just fine with the 12-ounce bottles, but you may need to use caution if you’re using the larger bottles and lots of mentos.
For the small bottles that we used, you’ll only need 1-2 Mentos tablets.
If you plan to try this experiment with your own kids, safety goggles are recommended. I had already tried it myself before my son got home, so I knew what size of an eruption to expect.
Here’s what we learned about the experiment:
- The reaction is a physical rather than chemical reaction . Dropping the Mentos into the soda breaks the carbon dioxide’s bond with water, allowing the CO2 to react with itself, causing the foam to escape.
- The speed at which you can drop the Mentos into the soda affects the size of the eruption.
- Use more Mentos for a bigger geyser. If you can drop a few in very quickly , your eruption will be larger than one with a single candy.
- Use bottles of various sizes to see different sizes of geysers.
- You don’t have to use Diet Coke! Any soda will do.
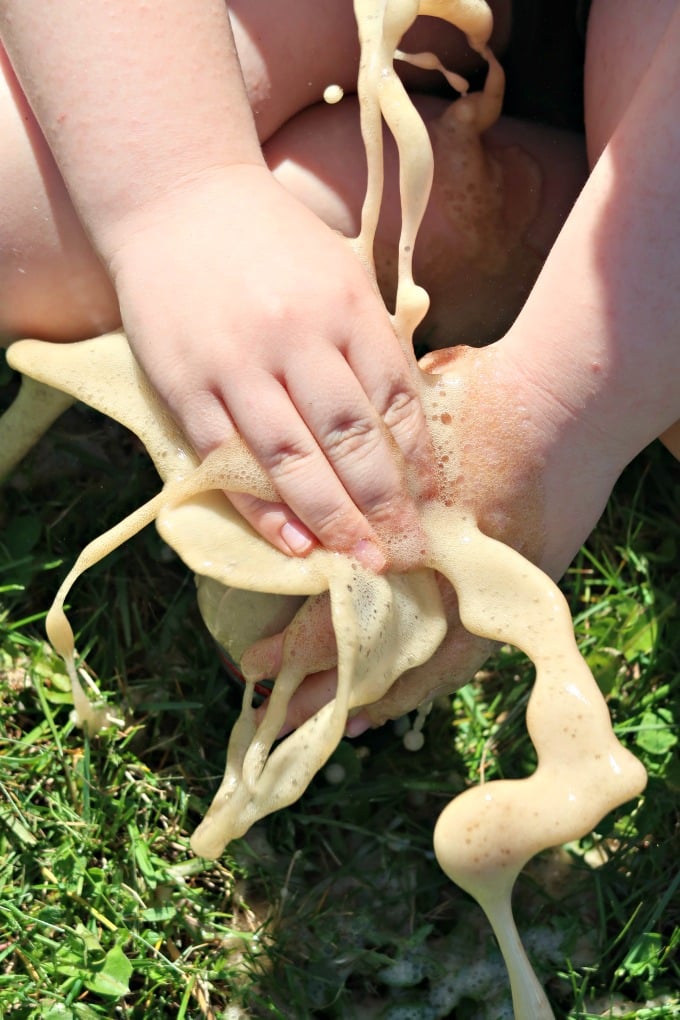
Do you plan to try the Mentos and Diet Coke experiment? We’d love to see your pictures or videos if you do!
For more kid-friendly science experiments, check out these posts:

35 Valentine Science Activities Kids Will Love

28 St. Patrick’s Day Science Activities
I'm Donella, the voice, heart, and wit (sometimes) behind this blog. I homeschool my pre-teen son by day and moonlight as a blogger and freelance writer. I'm a Diet Pepsi aficionado with a bookshelf that's always overflowing. My two dogs—a German Shepherd and a Beagle—are my fluffy shadows. I love planning in my bullet journal almost as much as I love hoarding notebooks and pens. I may be an introvert who missed her calling as a desert hermit, but that just gives me more time to write, right?

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Diet Coke and Mentos Soda Geyser

The Diet Coke and Mentos soda geyser, also known as the soda and candy fountain or volcano, is a physical reaction between candy and carbon dioxide that instantaneously releases the gas so it shoots into the air. There is a lot of science behind this deceptively simple project! Here are instructions for performing the original project, tips for getting the tallest eruption, options for material substitutions, and a look at how the Diet Coke and Mentos geyser works.
All you need is a packet of Mentos candies and a 2-liter bottle of Diet Coke:
- Roll of Mentos candies
- 2-liter bottle of Diet Coke
Make sure the candy is fresh and the bottle of soda is unopened. Freshness matters!
You also need a way of delivering the candies into the soda. One method is just dropping the column from your hand, but rolling them into a paper or index card tube is more reliable. Stacking them into a test tube is another option.
Substitutions
While Mentos and Diet Coke work best, you have other options:
- Any carbonated beverage
- Any candy that stacks neatly into a column
- Coins, shot, or other small items that fit through the bottle opening
- Sand or salt instead of candy (which work quite well)
In general, diet carbonated beverages produce higher fountains than sugary ones. Also, they don’t produce a sticky mess. Uncarbonated beverages, like juice or water, do not work at all. Objects with smooth, flat surfaces (like coins) do not work nearly as well as other options.
How to Make the Diet Coke and Mentos Soda Geyser Erupt
The project is messy. You might want to step outdoors.
- Open the Mentos candies and stack them into a single column.
- Open the bottle of soda.
- Drop the column of candy into the bottle, all at once.
If you have more candy, you can repeat the eruption using the same bottle of soda. It won’t be quite as dramatic, but still works.
Tips for Getting the Biggest Eruption
- Diet Coke or other diet colas outperform any other drinks. There are a lot of potential reasons for this, mainly involving the effects of aspartame, potassium benzoate, and other ingredients on the surface tension and foaming capacity of the beverage. The worst carbonated beverages for this project are carbonated water and sparkling alcoholic drinks.
- The blue Mentos candies work better than other flavors. The fruity Mentos are reportedly the worst flavor. Freshly unwrapped candies are best. Old candy is not very effective, probably because humidity changes the candy surface.
- A 2-liter plastic bottle works better than any smaller bottle, whether it is plastic or glass.
- You get a better eruption at high altitude or low atmospheric pressure compared with sea level or other high pressure situation.
- Warm soda produces a higher fountain than cold soda.
How the Diet Coke and Mentos Experiment Works
The Diet Coke and Mentos eruption is a physical process more than a chemical reaction. The candy surface has many tiny imperfections and cavities, each only a micron or so in size. When you drop the Mentos into the soda there are numerous minute air bubbles stuck onto them. These little bubbles act as nucleation sites for rapid de-gassing of the soda:
CO 2 (aq) → CO 2 (g)
Because the nucleation sites lower the activation energy for bubble formation, you can say they catalyze the reaction.
The candies are dense enough that they sink to the bottom of the soda bottle, interacting with dissolved carbon dioxide as they fall. As carbon dioxide bubbles form, the gas is lighter than the liquid and the bubbles rise. As they rise, they expand. The pressure of the gas results in a quick release of pressure, making a geyser out of the soda. Ingredients in the partially-dissolved candy help the bubbles keep their shape and form a foam as the liquid ejects from the bottle.
Numerous investigations into why diet soda (especially cola) works better than sweetened soda or why Mentos works better than other candies answer some questions, but not all of them. The ingredients in the soda make a difference. However, which ones enhance bubble formation and which suppress it are unclear. The chemical composition of the candies likely contributes to bubble formation, but it’s really their surface structure that matters the most.
Turn the Science Project Into an Experiment
Performing the Diet Coke and Mentos project is easy, but turning the project into an experiment is also simple. Just find a variable under your control, predict the outcome from changing it, conduct an experiment that tests this hypothesis , and then analyze your results and see if your prediction was correct. Here are some ideas of variables you can explore:
- Is there an optimal number of candies for the best eruption?
- Compare different types of carbonated beverages. Do you think, for example, that Coke Zero performs as well as Diet Coke? Do other brands of diet cola perform as well?
- Explore the effect of soda temperature on fountain formation. If you see a difference, comparing chilled and warm soda, can you explain it ?
- Are there any candies that work as well as Mentos? In general, is there a way of predicting whether or not a particular kind of candy produces an eruption?
- What effect do you expect, if you add a bit of bubble solution or dishwashing liquid to the soda before adding the candy?
- Design different “candy delivery” systems. What are the pros and cons of each of them?
- Can you make a nozzle that reduces the diameter of the bottle? If so, what effect does this have on the height of the eruption?
Fun Facts About the Diet Coke and Mentos Project
- The original soda and candy fountain project, circa 1910, used Wint-O-Green Lifesaver candies (which as also great for the “spark in the dark” triboluminescence project ). However, the company changed the candy diameter in the 1990s and it no longer fits into most bottles.
- Scientists estimate the eruption releases between 2.4 and 14 million bubbles per liter of Diet Coke. Regular Coke produces a lot less bubbles.
- A single Mentos candy contains 50,000 to 300,000 nucleation sites, although the reaction does not utilize every one of them.
- Coffey, Tonya Shea (2008). “Diet Coke and Mentos: What is really behind this physical reaction?”. American Journal of Physics . 76 (6): 551–557. doi: 10.1119/1.2888546
- Kuntzleman, Thomas S.; Imhoff, Amanda M. (2021). “How Many Bubbles Are in the Foam Produced during the Candy-Cola Soda Geyser?”. Journal of Chemical Education . 98 (12): 3915–3920. doi: 10.1021/acs.jchemed.1c01001
- Kuntzleman, Thomas S.; Annis, Jezrielle; Anderson, Hazel; Kenney, Joshua B.; Doctor, Ninad (2020). “Kinetic Modeling of and Effect of Candy Additives on the Candy–Cola Soda Geyser: Experiments for Elementary School Science through Physical Chemistry”. Journal of Chemical Education . 97 (1): 283–288. doi: 10.1021/acs.jchemed.9b00796
- Kuntzleman, Thomas S.; Johnson, Ryan (2020). “Probing the Mechanism of Bubble Nucleation in and the Effect of Atmospheric Pressure on the Candy–Cola Soda Geyser”. Journal of Chemical Education . 97 (4): 980–985. doi: 10.1021/acs.jchemed.9b01177
Related Posts
Leave a reply cancel reply.
This site uses Akismet to reduce spam. Learn how your comment data is processed .

IMAGES
COMMENTS
The Mentos And Coke Volcano Experiment. Now it's time to actually run the experiment, but first, we need to make a hypothesis. The Hypothesis. The scientific method is an important way scientists make observations and come to conclusions.. Part of the scientific method is making a prediction called a hypothesis.. Write down what you think will happen when placing the Mentos in the soda bottles.
Grab Some Mentos and Coke. Our Mentos and soda experiment is a fun example of a physical reaction. Read on to learn more about how this Mentos and Coke reaction works. We love fizzing experiments and have been exploring science for kindergarten, preschool, and early elementary for over 8 years now. Make sure to check out our collection of simple science experiments for kids.
Sometimes you'll see it called a coke and mento geyser, as the eruption looks like a geyser! Andernach Geyser Coke and Mentos Experiment You'll need: Coke or other fizzy soda. Mentos. Instructions . We dropped two Mentos into a bottle of normal Cola and Diet Cola. I used the cheapest brands available in our local supermarket.
Use the hot glue gun to glue 7 - 8 mentos together. This is optional, it just helps to add all of the mentos into the bottle at the same time. Place your bottle of coke in a large tub. This is optional, it just makes cleaning up more easy. Drop your mentos tower into the bottle. Stand back and enjoy.
This leads to the classic Mentos and Diet Coke eruption. The speed at which the Mentos falls through the Diet Coke can affect how large the eruption is. Because Mentos candies are rather dense, they sink rapidly through the bottle, causing a fast, large eruption. However, since there are air spaces between the small pieces, the group of crushed ...
Wrap the paper around the pack of Mentos to make a tube. Use masking tape to tape the tube closed. Remove the pack of Mentos from the tube. 3. Close off one end of the tube by cutting a little circle or square of paper and taping it to one end of the tube. 4. Open the pack of Mentos and place all of them in the tube.
This video shows how to do the Mentos and Coke experiment using just a few simple ingredients and supplies. Materials Needed. To do the Mentos and Coke experiment, you will need: A roll or box of mint-flavored Mentos; 2-liter bottle of Coca-Cola (aka Coke) Sheet of paper to roll into a tube OR pre-made geyser tube; Tape
In the Diet Coke bottle the Mentos candy provides a rough surface that allows the bonds between the carbon dioxide gas and water to break more easily, helping to create carbon dioxide bubbles.
This classic Diet Coke and Mentos experiment is such fun! Create a small or big eruption of foam with candy and soda - see the results of our experiment here. Skip to Content. ... but you may need to use caution if you're using the larger bottles and lots of mentos. For the small bottles that we used, you'll only need 1-2 Mentos tablets.
A 2-liter plastic bottle works better than any smaller bottle, whether it is plastic or glass. You get a better eruption at high altitude or low atmospheric pressure compared with sea level or other high pressure situation. Warm soda produces a higher fountain than cold soda. How the Diet Coke and Mentos Experiment Works