What is the 'Gold Foil Experiment'? The Geiger-Marsden experiments explained
Physicists got their first look at the structure of the atomic nucleus.


J.J. Thomson model of the atom
Gold foil experiments, rutherford model of the atom.
- The real atomic model
Additional Resources
Bibliography.
The Geiger-Marsden experiment, also called the gold foil experiment or the α-particle scattering experiments, refers to a series of early-20th-century experiments that gave physicists their first view of the structure of the atomic nucleus and the physics underlying the everyday world. It was first proposed by Nobel Prize -winning physicist Ernest Rutherford.
As familiar as terms like electron, proton and neutron are to us now, in the early 1900s, scientists had very little concept of the fundamental particles that made up atoms .
In fact, until 1897, scientists believed that atoms had no internal structure and believed that they were an indivisible unit of matter. Even the label "atom" gives this impression, given that it's derived from the Greek word "atomos," meaning "indivisible."

But that year, University of Cambridge physicist Joseph John Thomson discovered the electron and disproved the concept of the atom being unsplittable, according to Britannica . Thomson found that metals emitted negatively charged particles when illuminated with high-frequency light.
His discovery of electrons also suggested that there were more elements to atomic structure. That's because matter is usually electrically neutral; so if atoms contain negatively charged particles, they must also contain a source of equivalent positive charge to balance out the negative charge.
By 1904, Thomson had suggested a "plum pudding model" of the atom in which an atom comprises a number of negatively charged electrons in a sphere of uniform positive charge, distributed like blueberries in a muffin.
The model had serious shortcomings, however — primarily the mysterious nature of this positively charged sphere. One scientist who was skeptical of this model of atoms was Rutherford, who won the Nobel Prize in chemistry for his 1899 discovery of a form of radioactive decay via α-particles — two protons and two neutrons bound together and identical to a helium -4 nucleus, even if the researchers of the time didn't know this.
Rutherford's Nobel-winning discovery of α particles formed the basis of the gold foil experiment, which cast doubt on the plum pudding model. His experiment would probe atomic structure with high-velocity α-particles emitted by a radioactive source. He initially handed off his investigation to two of his protégés, Ernest Marsden and Hans Geiger, according to Britannica .
Rutherford reasoned that if Thomson's plum pudding model was correct, then when an α-particle hit a thin foil of gold, the particle should pass through with only the tiniest of deflections. This is because α-particles are 7,000 times more massive than the electrons that presumably made up the interior of the atom.
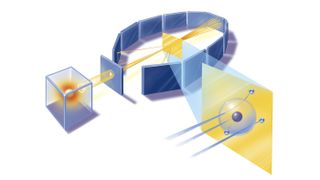
Marsden and Geiger conducted the experiments primarily at the Physical Laboratories of the University of Manchester in the U.K. between 1908 and 1913.
The duo used a radioactive source of α-particles facing a thin sheet of gold or platinum surrounded by fluorescent screens that glowed when struck by the deflected particles, thus allowing the scientists to measure the angle of deflection.
The research team calculated that if Thomson's model was correct, the maximum deflection should occur when the α-particle grazed an atom it encountered and thus experienced the maximum transverse electrostatic force. Even in this case, the plum pudding model predicted a maximum deflection angle of just 0.06 degrees.
Of course, an α-particle passing through an extremely thin gold foil would still encounter about 1,000 atoms, and thus its deflections would be essentially random. Even with this random scattering, the maximum angle of refraction if Thomson's model was correct would be just over half a degree. The chance of an α-particle being reflected back was just 1 in 10^1,000 (1 followed by a thousand zeroes).
Yet, when Geiger and Marsden conducted their eponymous experiment, they found that in about 2% of cases, the α-particle underwent large deflections. Even more shocking, around 1 in 10,000 α-particles were reflected directly back from the gold foil.
Rutherford explained just how extraordinary this result was, likening it to firing a 15-inch (38 centimeters) shell (projectile) at a sheet of tissue paper and having it bounce back at you, according to Britannica
Extraordinary though they were, the results of the Geiger-Marsden experiments did not immediately cause a sensation in the physics community. Initially, the data were unnoticed or even ignored, according to the book "Quantum Physics: An Introduction" by J. Manners.
The results did have a profound effect on Rutherford, however, who in 1910 set about determining a model of atomic structure that would supersede Thomson's plum pudding model, Manners wrote in his book.
The Rutherford model of the atom, put forward in 1911, proposed a nucleus, where the majority of the particle's mass was concentrated, according to Britannica . Surrounding this tiny central core were electrons, and the distance at which they orbited determined the size of the atom. The model suggested that most of the atom was empty space.
When the α-particle approaches within 10^-13 meters of the compact nucleus of Rutherford's atomic model, it experiences a repulsive force around a million times more powerful than it would experience in the plum pudding model. This explains the large-angle scatterings seen in the Geiger-Marsden experiments.
Later Geiger-Marsden experiments were also instrumental; the 1913 tests helped determine the upper limits of the size of an atomic nucleus. These experiments revealed that the angle of scattering of the α-particle was proportional to the square of the charge of the atomic nucleus, or Z, according to the book "Quantum Physics of Matter," published in 2000 and edited by Alan Durrant.
In 1920, James Chadwick used a similar experimental setup to determine the Z value for a number of metals. The British physicist went on to discover the neutron in 1932, delineating it as a separate particle from the proton, the American Physical Society said .
What did the Rutherford model get right and wrong?
Yet the Rutherford model shared a critical problem with the earlier plum pudding model of the atom: The orbiting electrons in both models should be continuously emitting electromagnetic energy, which would cause them to lose energy and eventually spiral into the nucleus. In fact, the electrons in Rutherford's model should have lasted less than 10^-5 seconds.
Another problem presented by Rutherford's model is that it doesn't account for the sizes of atoms.
Despite these failings, the Rutherford model derived from the Geiger-Marsden experiments would become the inspiration for Niels Bohr 's atomic model of hydrogen , for which he won a Nobel Prize in Physics .
Bohr united Rutherford's atomic model with the quantum theories of Max Planck to determine that electrons in an atom can only take discrete energy values, thereby explaining why they remain stable around a nucleus unless emitting or absorbing a photon, or light particle.
Thus, the work of Rutherford, Geiger (who later became famous for his invention of a radiation detector) and Marsden helped to form the foundations of both quantum mechanics and particle physics.
Rutherford's idea of firing a beam at a target was adapted to particle accelerators during the 20th century. Perhaps the ultimate example of this type of experiment is the Large Hadron Collider near Geneva, which accelerates beams of particles to near light speed and slams them together.
- See a modern reconstruction of the Geiger-Marsden gold foil experiment conducted by BackstageScience and explained by particle physicist Bruce Kennedy .
- Find out more about the Bohr model of the atom which would eventually replace the Rutherford atomic model.
- Rutherford's protege Hans Gieger would eventually become famous for the invention of a radioactive detector, the Gieger counter. SciShow explains how they work .
Thomson's Atomic Model , Lumens Chemistry for Non-Majors,.
Rutherford Model, Britannica, https://www.britannica.com/science/Rutherford-model
Alpha particle, U.S NRC, https://www.nrc.gov/reading-rm/basic-ref/glossary/alpha-particle.html
Manners. J., et al, 'Quantum Physics: An Introduction,' Open University, 2008.
Durrant, A., et al, 'Quantum Physics of Matter,' Open University, 2008
Ernest Rutherford, Britannica , https://www.britannica.com/biography/Ernest-Rutherford
Niels Bohr, The Nobel Prize, https://www.nobelprize.org/prizes/physics/1922/bohr/facts/
House. J. E., 'Origins of Quantum Theory,' Fundamentals of Quantum Mechanics (Third Edition) , 2018
Sign up for the Live Science daily newsletter now
Get the world’s most fascinating discoveries delivered straight to your inbox.
Robert Lea is a science journalist in the U.K. who specializes in science, space, physics, astronomy, astrophysics, cosmology, quantum mechanics and technology. Rob's articles have been published in Physics World, New Scientist, Astronomy Magazine, All About Space and ZME Science. He also writes about science communication for Elsevier and the European Journal of Physics. Rob holds a bachelor of science degree in physics and astronomy from the U.K.’s Open University
Do atoms ever touch?
Could monkeys really type the complete works of Shakespeare?
Bat poop used to grow cannabis kills 2 in New York in unusual cases

Most Popular
- 2 Radiation-resistant 'extremophile' microbe dubbed 'Conan the Bacterium' inspires new antioxidant
- 3 Henry the giant crocodile, who has sired 10,000 babies, celebrates 124th birthday
- 4 World's 1st nuclear-diamond battery of its kind could power devices for 1000s of years
- 5 After accident crash on Mars, NASA's Ingenuity helicopter could live on as a weather station for 20 years
About Rutherford's Gold Foil Experiment

Ernest Rutherford, originally from New Zealand, is credited as being the father of nuclear physics for his discoveries in atomic structure, even though Hantaro Nagaoka, a physicist from the Imperial University of Tokyo, first proposed the theory of the nucleus as it is known today. Rutherford's "gold foil experiment" led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, Rutherford was granted the Nobel Prize for other key contributions in the field of chemistry.
The popular theory of atomic structure at the time of Rutherford's experiment was the "plum pudding model." This model was developed in 1904 by J.J. Thompson, the scientist who discovered the electron. This theory held that the negatively charged electrons in an atom were floating in a sea of positive charge–the electrons being akin to plums in a bowl of pudding. Although Dr. Nagaoka had published his competing theory that electrons orbit a positive nucleus, akin to the way the planet Saturn is orbited by its rings, in 1904, the plum pudding model was the prevailing theory on the structure of the atom until it was disproved by Ernest Rutherford in 1911.
The gold foil experiment was conducted under the supervision of Rutherford at the University of Manchester in 1909 by scientist Hans Geiger (whose work eventually led to the development of the Geiger counter) and undergraduate student Ernest Marsden. Rutherford, chair of the Manchester physics department at the time of the experiment, is given primary credit for the experiment, as the theories that resulted are primarily his work. Rutherford's gold foil experiment is also sometimes referred to as the Geiger-Marsden experiment.
The gold foil experiment consisted of a series of tests in which a positively charged helium particle was shot at a very thin layer of gold foil. The expected result was that the positive particles would be moved just a few degrees from their path as they passed through the sea of positive charge proposed in the plum pudding model. The result, however, was that the positive particles were repelled off of the gold foil by nearly 180 degrees in a very small region of the atom, while most of the remaining particles were not deflected at all but rather passed right through the atom.
Significance
The data generated from the gold foil experiment demonstrated that the plum pudding model of the atom was incorrect. The way in which the positive particles bounced off the thin foil indicated that the majority of the mass of an atom was concentrated in one small region. Because the majority of the positive particles continued on their original path unmoved, Rutherford correctly deducted that most of the remainder of the atom was empty space. Rutherford termed his discovery "the central charge," a region later named the nucleus.
Rutherford's discovery of the nucleus and proposed atomic structure was later refined by physicist Niels Bohr in 1913. Bohr's model of the atom, also referred to as the Rutherford Bohr model, is the basic atomic model used today. Rutherford's description of the atom set the foundation for all future atomic models and the development of nuclear physics.
Cite This Article
Pestka, Jessica. "About Rutherford's Gold Foil Experiment" sciencing.com , https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/. 24 April 2017.
Pestka, Jessica. (2017, April 24). About Rutherford's Gold Foil Experiment. sciencing.com . Retrieved from https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/
Pestka, Jessica. About Rutherford's Gold Foil Experiment last modified March 24, 2022. https://www.sciencing.com/rutherfords-gold-foil-experiment-4569065/
Recommended

IMAGES
VIDEO