An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Cloning of breeding buffalo bulls in India: Initiatives & challenges
Naresh l selokar.
- Author information
- Article notes
- Copyright and License information
For correspondence: Dr Naresh L. Selokar, Division of Animal Physiology & Reproduction, ICAR-Central Institute for Research on Buffaloes, Hisar 125 001, Haryana, India e-mail: [email protected]
Received 2017 Dec 31.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
The term animal cloning refers to an asexual mean of reproduction to produce genetically identical copies of any animal without the use of sperm. In India, the cloning of buffalo is well established and clones of the Murrah, the best dairy breed of buffalo, have been produced. The most acclaimed example is the restoration of progeny-tested breeding bull by isolating somatic cells from frozen doses of semen, which were stored for more than a decade in the semen bank. Buffalo bull cloning is considered the best available option to reproduce declared proven bulls and their semen would contribute to accomplishing the demand of ever-growing frozen semen, which is the prime requirement of conventional breeding. This article highlights the importance of buffalo bull cloning and its current status in India.
Keywords: Animal cloning, buffalo, bull, cloned embryo, semen
Introduction
In India, domestic buffalo ( Bubalus bubalis ) has been contributing to country's milk and meat production and is a source of employment to the millions of farmers. In 2016-2017, India produced 165.4 million tons of milk, of which approximately 49 per cent (81 million tons) was contributed by the buffalo (Annual Report 2017-2018, Department of Animal Husbandry Dairying and Fisheries (DADF) of India 1 . In addition to milk, the country had exported buffalo meat and meat products to the world worth of ₹4036.89 USD millions in 2017-2018, which makes buffalo the preferred bovine animal to boost the Indian meat industry (data were obtained from the Agricultural and Processed Food Products Export Development Authority of India 2 . Overall, buffalo is considered as the best animal of choice for India's dairy and meat sector. In spite of its huge contribution to milk production and meat export, less efforts have been invested to improve buffalo's genetic potential for higher milk and meat productivity, particularly the use of assisted reproductive techniques (ARTs) such as superovulation, embryo transfer technology, ovum pickup, in vitro fertilization (IVF) and somatic cell nuclear transfer (SCNT) 3 , 4 . Over the many decades, the artificial insemination (AI) has been used to improve the buffalo germplasm, and it will continue to contribute because there is no replacement of AI which can easily be adopted at the village level. In the last decade, there has been increasing interest to explore in vitro embryo production (IVEP) technologies and SCNT for the faster propagation of superior buffalo germplasm. In the buffalo breeding, advanced ARTs are already in place coupled with conventional breeding programmes to improve the germplasm.
SCNT, commonly known as animal cloning, is an advanced ART technique used to multiply the best productive animals. Many species of domestic animals (cattle, buffalo, pig, sheep, goat, camel and horse) have already been cloned using SCNT methods 5 . In the last few years, the clones of elite breeding bulls were produced by our group and their semen was used for AI and produced normal healthy calves 4 , 6 . We earlier reported birth of a cloned bull by isolating somatic cells from frozen semen that were stored for a decade in the semen bank 7 . This report has opened a new way to restore the population of superior progeny-tested bulls, who died or culled, but their semen was available in the semen banks. Despite tremendous application of buffalo cloning, the technique is suffered from lower pregnancy rates, high abortion rates and poor survival of clones 6 . This article highlights the present status of buffalo bull cloning in India and its challenges.
Why breeding bulls only to be cloned?
The importance of bulls is often underestimated in breeding programmes and more focus has been given to female animals to boost herd productivity. In the herd, one female is responsible for half genetic of one calf per year, whereas one bull can be responsible for half genetic of a large number of calves. Conventionally, the genetic improvement in domestic animals has been achieved by inseminating females with the semen of desirable genotype bulls to produce higher productive calves 8 . Therefore, availability of best genotype bulls is a fundamental requirement to boost genetic gains. In India, the National Dairy Plan (NDP) has been initiated to increase the productivity of dairy animals, and it has been anticipated that higher productivity can be achieved by expanding coverage of AI. According to the NDP strategy, during the first phase, around 35 per cent of breedable bovines (cattle and buffalo) need to be covered under AI (ongoing), and 50 per cent population need to be covered by the end of the second phase (2021-2022), from an existing 15 to 20 per cent coverage of AI 9 . According to the NDP estimates, the requirement of semen doses to achieve targets would be approximately 100 million frozen doses annually (900 bulls) during the first phase and 140 million frozen doses annually (1200 bulls) by the end of the second phase 9 .
In India, the majority of domestic animal bulls, particularly buffalo and cattle, are selected on the basis of their pedigree records followed by extensive progeny testing programmes (a bull to be declared proven requires the span of 8 to 10 years, and by that time either bull is culled or limited semen doses are available in the semen bank). In addition to in-house bull production, the country has also been importing exotic bulls and equivalent embryos to fulfill the demand of bulls for breeding. During 2016-17, 400 exotic bulls and/or equivalent embryos were imported to accomplish demand of dairy bulls in the country 1 .
SCNT can also be used to produce breeding bulls to reduce dependency on other methods of bull production and procurement (a schematic presentation of bull cloning for buffalo breeding is depicted in the Figure ). Advantages of the bull cloning over to the female cloning are ( i ) bulls have a significant direct impact on the genetic gain in the population within a short period of time; ( ii ) a number of identical clone copies can be produced from a proven bull to compensate demand of its semen; and ( iii ) due to lower efficiency of cloning, approximately <2 per cent, it is practically impossible to produce a huge number of females, but hundreds of elite bulls can be cloned. If the best genetic merit breeding bull was culled from semen production due to ageing or infection or lameness or any reason and limited semen doses are available in the semen bank, cloning can be done.
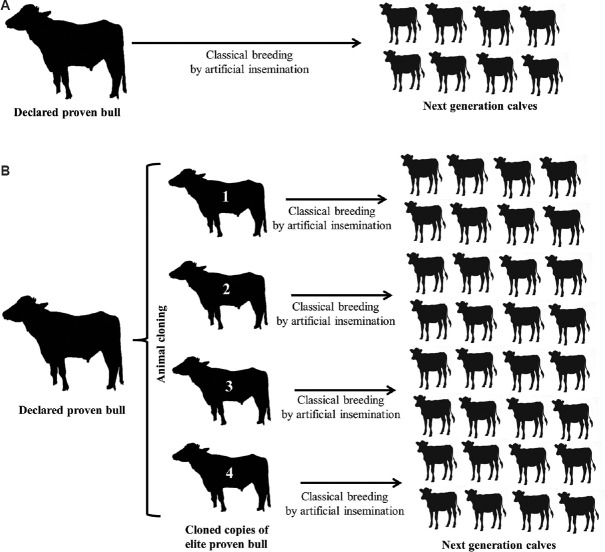
Schematic presentation of how the buffalo bull cloning can be included in the buffalo breeding programme along with the classical breeding. Conventionally, the bull is nominated for breeding after rigorous and exhaustive progeny testing studies, which takes 8-10 years to declare the bull is proven, after the nomination, semen doses of the proven bull are used to inseminate females to produce future elite calves ( A ). If such proven bull (s) died or culled and have a very few doses of semen, then farmers and breeders could not have a constant supply of proven semen for breeding. Through animal cloning, it is possible to restore the genotype of declared proven bulls and the semen of such restored bulls can mitigate the demand of proven semen for classical breeding ( B ). This way, farmers and breeders can speed up the genetic gain. Also, multiple locations or herds can be targeted by cloned copies of one bull to disseminate best genetic at large scale.
Previous studies in cattle and pigs suggested that cloned bulls have normal production and reproduction performances such as the production of good quality semen and ability to impregnate females 10 , 11 . Furthermore, several reports demonstrated that the progenies of clones exhibited normal growth, health, reproduction and production similar to animals produced through conventional breeding 12 , 13 , 14 , 15 . By considering the requirement of buffalo bulls in the country, more efforts are needed to produce more cloned copies of bulls, particularly declared proven bulls.
Current status of buffalo bull cloning in India
Animal cloning in India was first attempted in buffalo 1990s 16 . During that period, the technique used to clone the buffalo was similar to micromanipulator-assisted SCNT. Despite the multiple attempts, researchers could not produce the blastocyst stage embryos 16 . Later the micromanipulator-free SCNT technique called handmade cloning (HMC) was comprehensively demonstrated in cattle by Vajta et al 17 , India's first live cloned buffalo was born in 2009, and afterward, more than 20 buffalo clones, including breeding bulls, were produced in India 4 , 6 . Saini et al 6 reviewed that cloned male buffaloes donated semen was used for AI which resulted in the production of healthy calves. This indicates that cloned bulls can be used along with conventionally produced bulls to disseminate elite germplasm using application of ARTs (AI, IVF and multiple ovulation and embryo transfer). In addition, the female clones delivered the progenies following AI 6 .
Challenges of buffalo cloning research
Buffalo cloning offers a great hope for conventional breeding to speed up the dissemination of elite germplasm, particularly bulls; however, the results are not always promising because the cloning efficiency has improved marginally over the past decade, and poor conception rates, early pregnancy losses and mortality of born clones are the major challenges. Extensive research of cattle and pig cloning indicates that major factor which affects the efficiency of cloning is the abnormal nuclear reprogramming. Abnormal epigenetic modifications such as aberrant DNA methylation and histone modifications were also observed in cloned buffalo embryos 6 . Therefore, epigenetic errors need to be controlled to improve the buffalo cloning success rates. Our research team has investigated the faulty reprogramming of donor nucleus to better understand the cellular and molecular mechanisms of cloned embryonic development in buffalo 18 , 19 , 20 , 21 . However, the exact cause of faulty reprogramming is not yet identified and in vitro improvements did not translate to in vivo success; therefore, more efforts in this direction are needed. On the basis of our previous experiments 4 , 6 , several approaches such as selection of competent oocytes using Brilliant Cresyl Blue staining, culture of embryos under lower oxygen tension, treatment of embryos with epigenetic modifiers and modification in embryo production method like single ooplasm to avoid mitochondrial heteroplasmy, can be used to improve embryo quality to achieve more pregnancies.
It was noticed that approximately 50 per cent of buffalo clones died during the first three months after birth and they displayed various abnormalities such as enlarged umbilical cord, abnormal respiration, abnormal large size body organs and succumbed to infections 6 . Therefore, the practicing veterinarians can play an important role to develop preventive measures and care for newborn clones, so that they can survive long.
In addition to IVEP and refinement of the technique, the stringent selection of best embryo and best recipient for ET is needed to achieve higher pregnancy rates and overall success. A previous study suggested that the selection of healthy reproductive animals for embryo transfer is crucial and necessary to rule out the possibilities of lower conception rates due to inferior recipients 22 . At present, in India we neither have a large number of cloned embryo transfer data to predict conception rates nor many cloned animals to predict their performances such as milk production ability of females and fertility of bulls; therefore, it would be premature to interpret the exact efficiency and success of buffalo cloning in India. Therefore, consistent efforts are needed to generate buffalo specific data, which can be explored to improve buffalo cloning efficiency.
Breeding bulls can multiply using SCNT techniques, including HMC. Demand for semen doses of progeny-tested bulls is high among the farmers and breeders due to their high breeding merits. Some of the tested bulls have died or culled, and their limited semen doses are available in the semen banks. It is possible to clone these bulls through SCNT by isolating somatic cells from frozen semen.
Financial support & sponsorship:
Buffalo cloning work in India was supported by the Indian Council of Agricultural Research; Department of Biotechnology; Department of Science and Technology, New Delhi; and Chhattisgarh Wildlife Department, Raipur.
Conflicts of Interest:
Acknowledgment.
The author acknowledges the support from members of cloning teams from ICAR- National Dairy Research Institute, Karnal and ICAR-Central Institute for Research on Buffaloes (CIRB), Hisar, Haryana. The author thanks Prof. Suresh Singla, who was the author's mentor for Ph.D. studies, for his valuable guidance and technical support to pursue buffalo cloning research at the CIRB.
- 1. Annual Report 2017- 2018. New Delhi: Ministry of Agriculture & Farmers Welfare, Government of India; [accessed on November 24, 2018]. Department of Animal Husbandry, Dairying & Fisheries. Available from: http://dahd.nic.in/sites/default/filess/annual_report_17-18. pdf . [ Google Scholar ]
- 2. Buffalo meat. New Delhi: Ministry of Commerce & Industry, Government of India; [accessed on December 31, 2017]. Agricultural & Processed Food Products Export Development Authority. Available from: http://apeda. gov.in/apedawebsite/SubHead_Products/Buffalo_Meat.htm . [ Google Scholar ]
- 3. Singla SK, Selokar NL, Saini M, Palta P, Chauhan MS, Manik RS. Buffalo cloning: What we have achieved so far. Curr Sci. 2015;109:670–1. [ Google Scholar ]
- 4. Selokar NL, Saini M, Palta P, Chauhan MS, Manik RS, Singla SK. Cloning of buffalo, a highly valued livestock species of South and Southeast Asia: Any achievements? Cell Reprogram. 2018;20:89–98. doi: 10.1089/cell.2017.0051. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Keefer CL. Artificial cloning of domestic animals. Proc Natl Acad Sci U S A. 2015;112:8874–8. doi: 10.1073/pnas.1501718112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Saini M, Selokar NL, Palta P, Chauhan MS, Manik RS, Singla SK. An update: Reproductive handmade cloning of water buffalo (Bubalus bubalis) Anim Reprod Sci. 2018;197:1–9. doi: 10.1016/j.anireprosci.2018.08.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Selokar NL, Saini M, Palta P, Chauhan MS, Manik R, Singla SK. Hope for restoration of dead valuable bulls through cloning using donor somatic cells isolated from cryopreserved semen. PLoS One. 2014;9:e90755. doi: 10.1371/journal.pone.0090755. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 8. García-Ruiz A, Cole JB, VanRaden PM, Wiggans GR, Ruiz-López FJ, Van Tassell CP. Changes in genetic selection differentials and generation intervals in US Holstein dairy cattle as a result of genomic selection. Proc Natl Acad Sci U S A. 2016;113:E3995–4004. doi: 10.1073/pnas.1519061113. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 9. Project Management Unit. National Dairy Development Board. National Dairy Plan Phase I. Manual on Semen Production. Project Implementation Plan: Volume IV C. Anand: NDDB. [accessed on December 31, 2017]. Available from: https://www.nddb.coop/ sites/default/files/pdfs/guidelines/PIP-Vol-IV-C-Manual-on- Semen-Production.pdf .
- 10. Mir B, Zaunbrecher G, Archer GS, Friend TH, Piedrahita JA. Progeny of somatic cell nuclear transfer (SCNT) pig clones are phenotypically similar to non-cloned pigs. Cloning Stem Cells. 2005;7:119–25. doi: 10.1089/clo.2005.7.119. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Tecirlioglu RT, Cooney MA, Korfiatis NA, Hodgson R, Williamson M, Downie S, et al. Semen and reproductive profiles of genetically identical cloned bulls. Theriogenology. 2006;65:1783–99. doi: 10.1016/j.theriogenology.2005.09.033. [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Enright BP, Taneja M, Schreiber D, Riesen J, Tian XC, Fortune JE, et al. Reproductive characteristics of cloned heifers derived from adult somatic cells. Biol Reprod. 2002;66:291–6. doi: 10.1095/biolreprod66.2.291. [ DOI ] [ PubMed ] [ Google Scholar ]
- 13. Savage AF, Maull J, Tian XC, Taneja M, Katz L, Darre M, et al. Behavioral observations of adolescent Holstein heifers cloned from adult somatic cells. Theriogenology. 2003;60:1097–110. doi: 10.1016/s0093-691x(03)00110-9. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Heyman Y, Richard C, Rodriguez-Martinez H, Lazzari G, Chavatte-Palmer P, Vignon X, et al. Zootechnical performance of cloned cattle and offspring: Preliminary results. Cloning Stem Cells. 2004;6:111–20. doi: 10.1089/1536230041372364. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Williams NE, Walker SC, Reeves DE, Sherrer E, Galvin JM, Polejaeva I, et al. A comparison of reproductive characteristics of boars generated by somatic cell nuclear transfer to highly related conventionally produced boars. Cloning Stem Cells. 2006;8:130–9. doi: 10.1089/clo.2006.8.130. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Singla SK, Manik RS, Madan ML. Micromanipulation and cloning studies on buffalo oocytes and embryos using nucleus transfer. Indian J Exp Biol. 1997;35:1273–83. [ PubMed ] [ Google Scholar ]
- 17. Vajta G, Lewis IM, Hyttel P, Thouas GA, Trounson AO. Somatic cell cloning without micromanipulators. Cloning. 2001;3:89–95. doi: 10.1089/15204550152475590. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Saini M, Selokar NL, Agrawal H, Singla SK, Chauhan MS, Manik RS, et al. Treatment of buffalo (Bubalus bubalis) donor cells with trichostatin A and 5-aza-2’-deoxycytidine alters their growth characteristics, gene expression and epigenetic status and improves the in vitro developmental competence, quality and epigenetic status of cloned embryos. Reprod Fertil Dev. 2016;28:824–37. doi: 10.1071/RD14176. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Saini M, Selokar NL, Agrawal H, Singla SK, Chauhan MS, Manik RS, et al. Treatment of donor cells and reconstructed embryos with a combination of trichostatin-A and 5-aza-2’-deoxycytidine improves the developmental competence and quality of buffalo embryos produced by handmade cloning and alters their epigenetic status and gene expression. Cell Reprogram. 2017;19:208–15. doi: 10.1089/cell.2016.0061. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Mohapatra SK, Sandhu A, Neerukattu VS, Singh KP, Selokar NL, Singla SK, et al. Buffalo embryos produced by handmade cloning from oocytes selected using brilliant cresyl blue staining have better developmental competence and quality and are closer to embryos produced by in vitro fertilization in terms of their epigenetic status and gene expression pattern. Cell Reprogram. 2015;17:141–50. doi: 10.1089/cell.2014.0077. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Selokar NL, Saini M, Agrawal H, Palta P, Chauhan MS, Manik R, et al. Valproic acid increases histone acetylation and alters gene expression in the donor cells but does not improve the in vitro developmental competence of buffalo (Bubalus bubalis) embryos produced by hand-made cloning. Cell Reprogram. 2017;19:10–8. doi: 10.1089/cell.2016.0029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Bauersachs S, Ulbrich SE, Zakhartchenko V, Minten M, Reichenbach M, Reichenbach HD, et al. The endometrium responds differently to cloned versus fertilized embryos. Proc Natl Acad Sci U S A. 2009;106:5681–6. doi: 10.1073/pnas.0811841106. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (739.5 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Sign up for alerts
- RESEARCH HIGHLIGHT
- 21 October 2019
Breeding buffalo bull cloned

The cloned bull at CIRB, Hisar. © CIRB
Indian researchers have successfully cloned a breeding bull of the milch Murrah buffalo 1 . Such cloning, they say, can be used as a valuable breeding tool to complement artificial insemination for improving buffalo germplasm in India and worldwide.
Despite several births of buffalo clones worldwide, no information is available on their health and reproductive performance. This is, however, essential if cloned bulls are to be employed in breeding schemes.
To determine the suitability of the cloned buffalo bulls for breeding schemes, scientists from India's ICAR-Central Institute for Research on Buffaloes in Hisar and the CSIR-Institute of Genomics and Integrative Biology in New Delhi, created embryos using skin- and semen-derived donor cells. They then transferred the embryos to buffaloes. Two of them became pregnant, of which gave birth to a healthy calf.
The cloned calf had no physical abnormalities in its face, eyes, nostrils and legs. Besides, its blood content and other biochemical parameters were within the normal reference ranges of the buffalo. The researchers say that the calf, now 40 months of age with body weight of 760 kilograms, is producing good quality semen.
Given the huge demand for quality semen, buffalo cloning can help produce breeding bulls to reduce dependency on conventional methods of bull production, says lead author Naresh L. Selokar. Such cloning, he says, could also help genetically improve the buffalo species.
1. Selokar, N. L. et al. Successful cloning of a superior buffalo bull. Sci. Rep. 9, 11366 (2019)
doi: https://doi.org/10.1038/nindia.2019.140
Postdoctoral fellowship in cancer biomarkers research
Postdoctoral fellowship in cancer biomarkers, center for primary health care research, Department of Clinical Sciences Malmö, Sweden.
Malmö (Stad), Skåne (SE)
Lund University
SCUT-GZIC Global Talent Recruitment
Faculty position at SCUT.
Guangzhou, Guangdong, China
Guangzhou International Campus, South China University of Technology
Tenure-track Faculty Positions in Particle Physics and Cosmology
Faculty Positions in Particle Physics and Cosmology
Hong Kong (HK)
Department of Physics, The Hong Kong University of Science & Technology (HKUST)

Publisher/Senior Publisher (Open access medical journals)
We are seeking a Publisher or Senior Publisher to manage a portfolio of open access proprietary medical journals in our division.
London, New York or Philadelphia – Hybrid working model
Springer Nature Ltd
Associate or Senior Editor (Sustainability- Political Science, Economics, Energy)
Title: Associate or Senior Editor (Sustainability- Political Science, Economics, Energy) Organization: Nature Communications Location: New York, ...
New York City, New York (US)
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Approaches used to improve epigenetic reprogramming in buffalo cloned embryos
Monika saini, naresh l selokar.
- Author information
- Article notes
- Copyright and License information
For correspondence: Dr Naresh L. Selokar, Division of Animal Physiology & Reproduction, ICAR-Central Institute for Research on Buffaloes, Hisar 125 001, Haryana, India e-mail: [email protected]
Received 2017 Dec 31.
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
The reproductive cloning in buffalo in India has been started using a simplified somatic cell nuclear transfer technique named handmade cloning. Since the birth of first cloned female buffalo in 2009, a number of buffalo clones have been produced in India by utilizing different types of donor cells such as ear cells, embryonic stem cells, semen somatic cells and urine somatic cells. The use of buffalo cloning on a large scale is restricted due to low pregnancy rates and poor calf survival. Considerable attempts have been made to improve the overall buffalo cloning efficiency, particularly by modifying epigenetic reprogramming of cloned embryos. Previous studies have demonstrated that chemical epigenetic modifiers such as trichostatin A and 5-aza-2’-deoxycytidine, m-carboxycinnamic acid bishydroxamide can be used to treat donor somatic cells and reconstructed fused embryos to correct the epigenetic reprogramming to enhance the overall cloning efficiency in terms of live birth rates.
Keywords: Birth rate, buffalo, cloned, embryos, epigenetics, reprogramming
Introduction
Somatic cell nuclear transfer (SCNT) is one of the most successful tools of assisted reproductive techniques, which has been used to produce identical copies of elite domestic animals such as cattle, buffalo and sheep, goats and pigs 1 , and for drug production (biopharming), regenerative medicine (nuclear stem cell lines to cure diseases) and conservation of genetic resources (cloning of endangered species) 2 . Buffalo SCNT has enormous potential applications; however, the technique has been suffering from low live offspring birth rate (<2%) after transfer of cloned blastocysts 3 , 4 . Many abnormalities such as high rate of early abortion, large offspring syndrome, placental defects and health issues of born clones have been reported in cloned animals 3 , 5 . These developmental abnormalities are conceivably accountable for poor cloning efficiency and it is believed that abnormal epigenetic reprogramming of differentiated somatic cells is the main cause of poor cloning efficiency across the species, including buffalo.
Incorrect or faulty epigenetic reprogramming of donor cells by recipient oocytes during SCNT is one of the key factors responsible for the low cloning efficiency; therefore, for achieving correct nuclear reprogramming, the epigenetic status of donor cells or cloned embryos needs to be manipulated in such a way that generated cloned blastocysts would be more closely similar to in vivo or in vitro fertilized (IVF) embryos. To correct the nuclear reprogramming and to improve the developmental competence of cloned embryos, various approaches have been employed either in somatic cells or in cloned embryos; however, significant success in the term of live birth rate has not been achieved. Here we discussed about in vitro studies in which epigenetic modifiers were used to correct reprogramming of SCNT buffalo embryos.
Methods of buffalo cloning
The buffalo can be cloned using two broadly used SCNT techniques, first is a classical SCNT in which micromanipulator instrument is used 6 ; whereas, other method is a micromanipulator-free SCNT called Handmade cloning (HMC) 7 , 8 . The HMC is preferred due to the low cost of embryo production and less requirement of highly skilled workforce to perform embryo manipulations. The HMC procedure includes five steps which are: ( i ) removal of genetic material (enucleation) from zona-free oocytes using a small blade, ( ii ) electro-fusion of donor cells with nucleus-free oocytes, ( iii ) activation of fused embryos, ( iv ) in vitro culture of activated embryo for 7 or 8 days, and ( v ) in vivo transfer of generated blastocysts into foster mothers to deliver cloned calves. Basic differences between classical SCNT and HMC are shown in Table I . The births of healthy calves have been reported in cattle, pig and goat and buffalo using HMC technique 3 .
Classical somatic cell nuclear transfer (SCNT) vs. simplified handmade cloning
Source: Ref. 8
Status of buffalo cloning in India
In India, a simplified HMC, which was earlier demonstrated by Vajta et al 9 in cattle has been adopted. The basic method was modified to support the developmental competence of buffalo oocytes and embryos, which resulted in the efficient enucleation, fusion and activation and high blastocyst production rates 7 , 10 . The first live birth of cloned buffalo was reported in 2009 using optimized buffalo HMC method 11 . Following the birth of first cloned buffalo, several cloned buffaloes were produced in India, and attempts are continued to produce more clones 3 . Optimized HMC technique has yielded much higher blastocyst production rate than that of IVF technique (35-45% blastocyst rates in case of HMC; whereas, 10-15% in case of IVF embryos); however, the large number of produced blastocysts could not be translated into live clones 3 . Previous studies in buffalo suggested that cloned embryos exhibited hypermethylated, abnormal histone acetylation and methylation and altered genes expression pattern as compared to IVF embryos 12 , 13 , 14 , 15 . These reports suggest that basic research is needed to understand the epigenetic machinery of somatic cells and cloned embryos to improve the success rate of SCNT.
Ways to correct epigenetic reprogramming
Different approaches have been used to correct the epigenetic reprogramming in cloned embryos such as treatment of somatic cells, oocytes and fused embryos with epigenetic modifiers or with oocytes or stem cells extract, overexpression or suppression of important regulatory genes in somatic cells or one cell stage cloned embryos ( Figure ). Buffalo somatic cells and/or fused embryos were treated with different epigenetic modulating agents, such as trichostatin A (TSA), 5-aza-2’-deoxycytidine (5-aza-dC), valproic acid (VPA) and m-carboxycinnamic acid bishydroxamide (CBHA) 14 , 16 , 17 , 18 . These studies showed that treatment of buffalo donor cells and/or one cell stage fused embryos or both with epigenetic modulators, alone or in combination, resulted in higher blastocyst production rates and lower level of apoptosis in generated cloned blastocysts 14 , 15 . Future studies are needed to transfer improved blastocysts produced following the treatment of epigenetic modifiers into recipients to improve live birth rates.
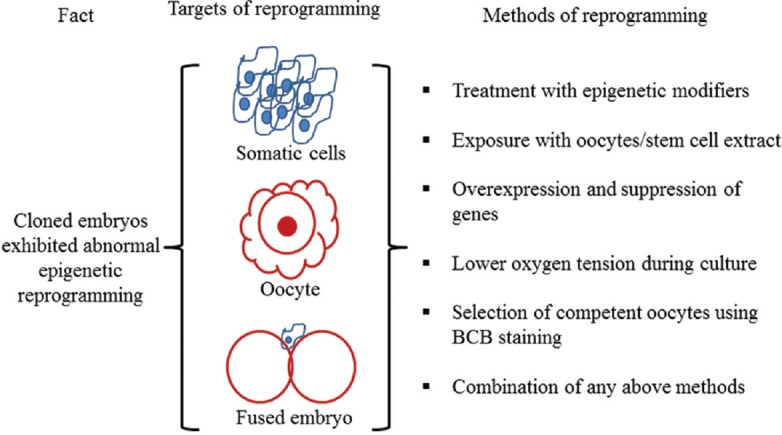
Different approaches used to correct an epigenetic reprogramming in buffalo cloned embryos. Somatic cell, oocyte, and fused embryo can be treated with any mentioned method either individually or in combination to improve reprogramming. BCB, Brilliant Cresyl Blue.
Source : Ref. 3 .
Use of epigenetic modifiers in buffalo cloning
Though different epigenetic modifiers were used to correct reprogramming in buffalo embryos; but best results were obtained with the combination of TSA and 5-aza-dC treatments ( Table II ). Panda et al 19 reported that treatment of fused embryos with 500 and 1000 nmol/l of scriptaid, a histone deacetylase inhibitor, significantly increased the blastocyst production rate. Furthermore, blastocysts derived from treated groups had higher cell number (339.9±1.4 and 343.4±2.4, respectively) than that of untreated group (150.7±2.0) 19 . Saini et al 14 examined the effects of treatment of donor cells with TSA and 5-aza-dC and found that donor cell treatments altered the expression of epigenetic-related genes, namely HDAC1, DNMT1 and DNMT3a . Treatment with these two epigenetic modifiers also increased the acetylation level of lysine at position 9 or 14 in histone 3 (H3K9/14ac), lysine at position 5 in histone 4 (H4K5ac), lysine at position 18 in histone 3 (H3K18ac) and decreased tri-methylation of lysine at position 27 in histone 3 (H3K27me3) in the cells. Simultaneous treatment of donor cells with TSA (50 nM) and 5-aza-dC (7.5 nM) resulted in improved blastocyst rates, lower apoptotic index and higher level of H3K27me3 in cloned blastocysts 14 . Subsequently, the same group 15 optimized doses of TSA and 5-aza-dC to improve reprogramming in buffalo cloned embryos by examining whether combined treatments of epigenetic modifiers would offer any advantage over treatment with the individual epigenetic modifier. Irrespective of whether donor cells or fused embryos or both treated with 50 nM TSA+7.5 nM 5-aza-dC, the blastocyst rates significantly improved ( Table I ); low apoptotic index, which was similar to blastocysts produced through in vitro fertilization; and higher level of H3K18ac and lower H3K27me3 level in blastocysts than that of untreated group. This study demonstrated that similar beneficial effects could be obtained by treatment of donor cells or fused embryos or both with 50 nM TSA+7.5 nM 5-aza-dC 15 . Selokar et al 16 used an epigenetic modifier, VPA, another class of histone deacetylase inhibitor, to treat the donor cells for correcting epigenetic reprogramming. This study demonstrated that treatment of donor cells with VPA did not improve the blastocyst production rate. Agrawal et al 17 reported that 10 μM of CBHA could be used to improve the blastocyst rates and quality of cloned embryos.
Epigenetic modifiers used in buffalo handmade cloning experiments
* Mentioned doses of TSA (trichostatin A) and 5-aza-dC (5-aza-2'-deoxycytidine) were optimized by treating somatic cells, fused embryos or both using different concentrations of these drugs. # Represents the significant improvement in blastocyst production rates than that of the untreated control group. SCNT, somatic cell nuclear transfer; CBHA, m-carboxycinnamic acid bishydroxamide
Beneficial effects are mediated by decreasing DNA and histone methylation and increasing histone acetylation. Treatment of buffalo donor cells or fused embryos or both with epigenetic modulators can be one of the ways to improve the success rate of buffalo SCNT. In addition to the use of epigenetic modifiers, other approaches have also been used such as lower oxygen tension during embryo culture 20 , use of BCB stating to screen best competent oocytes for the production of cloned embryos 21 . These approaches also resulted in an improvement in the blastocyst production rates and quality of produced blastocysts; however, more attempts are required to translate in vitro improvements into in vivo developments.
Buffalo cloning is a powerful reproductive tool to multiply elite animals, particularly proven bulls; however, live offspring production efficiency is low which is mainly due to abnormal epigenetic reprogramming. Previous studies demonstrated that abnormal epigenetic reprogramming can be corrected using the treatment of somatic cells or fused embryos with epigenetic modifiers, namely TSA and 5-aza-dC. In Buffalo, limited attempts were made to transfer epigenetically improved blastocysts into recipient animals to examine their in vivo developments; therefore, further studies are required to determine whether the beneficial effects observed in vitro following treatment of epigenetic modifiers would translate to live births.
Financial support & sponsorship:
This work was financially supported by the National Agriculture Innovative Project grants (C 2-1-(5)/2007, C-2067 and 075) to the ABTC, NDRI, Karnal, Haryana.
Conflicts of Interest:
Acknowledgment.
Authors thank the past members of buffalo cloning team of the Animal Biotechnology Centre (ABTC), National Dairy Research Institute (NDRI), Karnal, Haryana.
- 1. Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–3. doi: 10.1038/385810a0. [ DOI ] [ PubMed ] [ Google Scholar ]
- 2. Keefer CL. Artificial cloning of domestic animals. Proc Natl Acad Sci U S A. 2015;112:8874–8. doi: 10.1073/pnas.1501718112. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Saini M, Selokar NL, Palta P, Chauhan MS, Manik RS, Singla SK, et al. An update: Reproductive handmade cloning of water buffalo (Bubalus bubalis) Anim Reprod Sci. 2018;197:1–9. doi: 10.1016/j.anireprosci.2018.08.003. [ DOI ] [ PubMed ] [ Google Scholar ]
- 4. Selokar NL, Saini M, Palta P, Chauhan MS, Manik RS, Singla SK. Cloning of buffalo, a highly valued livestock species of South and Southeast Asia: Any achievements? Cell Reprogram. 2018;20:89–98. doi: 10.1089/cell.2017.0051. [ DOI ] [ PubMed ] [ Google Scholar ]
- 5. Yang F, Hao R, Kessler B, Brem G, Wolf E, Zakhartchenko V, et al. Rabbit somatic cell cloning: Effects of donor cell type, histone acetylation status and chimeric embryo complementation. Reproduction. 2007;133:219–30. doi: 10.1530/rep.1.01206. [ DOI ] [ PubMed ] [ Google Scholar ]
- 6. Shi D, Lu F, Wei Y, Cui K, Yang S, Wei J, et al. Buffalos (Bubalus bubalis) cloned by nuclear transfer of somatic cells. Biol Reprod. 2007;77:285–91. doi: 10.1095/biolreprod.107.060210. [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Shah RA, George A, Singh MK, Kumar D, Chauhan MS, Manik R, et al. Hand-made cloned buffalo (Bubalus bubalis) embryos: Comparison of different media and culture systems. Cloning Stem Cells. 2008;10:435–42. doi: 10.1089/clo.2008.0033. [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Vajta G. Handmade cloning: The future way of nuclear transfer? Trends Biotechnol. 2007;25:250–3. doi: 10.1016/j.tibtech.2007.04.004. [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Vajta G, Lewis IM, Hyttel P, Thouas GA, Trounson AO. Somatic cell cloning without micromanipulators. Cloning. 2001;3:89–95. doi: 10.1089/15204550152475590. [ DOI ] [ PubMed ] [ Google Scholar ]
- 10. Selokar NL, Shah RA, Saha AP, Muzaffar M, Saini M, Chauhan MS, et al. Effect of post-fusion holding time, orientation and position of somatic cell-cytoplasts during electrofusion on the development of handmade cloned embryos in buffalo (Bubalus bubalis) Theriogenology. 2012;78:930–6. doi: 10.1016/j.theriogenology.2012.03.018. [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Singla SK, Selokar NL, Saini M, Palta P, Chauhan MS, Manik R. Buffalo cloning: What we have achieved so far. Curr Sci. 2015;109:670–1. [ Google Scholar ]
- 12. Mohapatra SK, Sandhu A, Singh KP, Singla SK, Chauhan MS, Manik R, et al. Establishment of trophectoderm cell lines from buffalo (Bubalus bubalis) embryos of different sources and examination of in vitro developmental competence, quality, epigenetic status and gene expression in cloned embryos derived from them. PLoS One. 2015;10:e0129235. doi: 10.1371/journal.pone.0129235. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Jyotsana B, Sahare AA, Raja AK, Singh KP, Nala N, Singla SK, et al. Use of peripheral blood for production of buffalo (Bubalus bubalis) embryos by handmade cloning. Theriogenology. 2016;86:1318–240. doi: 10.1016/j.theriogenology.2016.04.073. [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Saini M, Selokar NL, Agrawal H, Singla SK, Chauhan MS, Manik RS, et al. Treatment of buffalo (Bubalus bubalis) donor cells with trichostatin A and 5-aza-2’-deoxycytidine alters their growth characteristics, gene expression and epigenetic status and improves the in vitro developmental competence, quality and epigenetic status of cloned embryos. Reprod Fertil Dev. 2016;28:824–37. doi: 10.1071/RD14176. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Saini M, Selokar NL, Agrawal H, Singla SK, Chauhan MS, Manik RS, et al. Treatment of donor cells and reconstructed embryos with a combination of trichostatin-A and 5-aza-2’-deoxycytidine improves the developmental competence and quality of buffalo embryos produced by handmade cloning and alters their epigenetic status and gene expression. Cell Reprogram. 2017;19:208–15. doi: 10.1089/cell.2016.0061. [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Selokar NL, Saini M, Agrawal H, Palta P, Chauhan MS, Manik R, et al. Valproic acid increases histone acetylation and alters gene expression in the donor cells but does not improve the in vitro developmental competence of buffalo (Bubalus bubalis) embryos produced by hand-made cloning. Cell Reprogram. 2017;19:10–8. doi: 10.1089/cell.2016.0029. [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Agrawal H, Selokar NL, Saini M, Singh MK, Chauhan MS, Palta P, et al. M-carboxycinnamic acid bishydroxamide improves developmental competence, reduces apoptosis and alters epigenetic status and gene expression pattern in cloned buffalo (Bubalus bubalis) embryos. Reprod Domest Anim. 2018;53:986–96. doi: 10.1111/rda.13198. [ DOI ] [ PubMed ] [ Google Scholar ]
- 18. Agrawal H, Selokar NL, Saini M, Singh MK, Chauhan MS, Palta P, et al. Epigenetic alteration of donor cells with histone deacetylase inhibitor m-carboxycinnamic acid bishydroxymide improves the in vitro developmental competence of buffalo (Bubalus bubalis) cloned embryos. Cell Reprogram. 2018;20:76–88. doi: 10.1089/cell.2017.0035. [ DOI ] [ PubMed ] [ Google Scholar ]
- 19. Panda SK, George A, Saha A, Sharma R, Singh AK, Manik RS, et al. Effect of scriptaid, a histone deacetylase inhibitor, on the developmental competence of handmade cloned buffalo (Bubalus bubalis) embryos. Theriogenology. 2012;77:195–200. doi: 10.1016/j.theriogenology.2011.07.033. [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Saini M, Selokar NL, Agrawal H, Singla SK, Chauhan MS, Manik RS, et al. Low oxygen tension improves developmental competence and reduces apoptosis in hand-made cloned buffalo (Bubalus bubalis) embryos. Livest Sci. 2015;172:106–9. [ Google Scholar ]
- 21. Mohapatra SK, Sandhu A, Neerukattu VS, Singh KP, Selokar NL, Singla SK, et al. Buffalo embryos produced by handmade cloning from oocytes selected using brilliant cresyl blue staining have better developmental competence and quality and are closer to embryos produced by in vitro fertilization in terms of their epigenetic status and gene expression pattern. Cell Reprogram. 2015;17:141–50. doi: 10.1089/cell.2014.0077. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (552.4 KB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES