Advertisement

Dynamic column breakthrough experiments for measurement of adsorption equilibrium and kinetics
- Published: 30 September 2020
- Volume 27 , pages 397–422, ( 2021 )
Cite this article

- Nicholas Stiles Wilkins 1 ,
- Arvind Rajendran 1 &
- Shamsuzzaman Farooq ORCID: orcid.org/0000-0002-6501-5540 2
7394 Accesses
75 Citations
5 Altmetric
Explore all metrics
This paper provides a set of comprehensive guidelines for the use of dynamic column breakthrough experiments to measure single and multi-component equilibria and kinetics. Recommendations are made for the design of the experimental rig, experimental procedures, and data processing methods to minimize errors and increase data reliability. Designing experiments to identify the transport mechanism, and quantitatively interpreting the breakthrough responses via modeling and optimization are also enumerated. Results reported in relevant published literature are also used to illustrate the ideas and concepts.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
The zero length column technique to measure adsorption equilibrium and kinetics: lessons learnt from 30 years of experience
Integrated uncertainty quantification and sensitivity analysis of single-component dynamic column breakthrough experiments
Equilibrium loadings and adsorption isotherm model parameters estimated from multi-component breakthrough curves
Abbreviations.
Cross-sectional area (m 2 )
Fluid concentration (mol/m 3 )
Heat capacity (J/mol/K)
Diameter (m)
Diffusivity (m 2 /s)
Convective heat transfer coefficient (W/m 2 /K)
Heat of adsorption (J/mol)
LDF coefficient (s −1 )
Henry’s constant (–) or thermal conductivity (W/m/K)
Molecular mass (g/mol)
Nusselt number (–)
Total pressure (Pa)
Peclet number (–)
Solid-phase loading (mol/m 3 )
Solid-phase equilibrium loading (mol/m 3 )
Standard flow (SLPM)
Radius or micropore position (m)
Macropore position (m)
Reynolds number (–)
Universal gas constant (Pa m 3 /mol/K)
Schmidt number (–)
Sherwood number (–)
Mean residence time (s)
Temperature (K)
Interstitial velocity (m/s)
Mole fraction (–)
Axial position (m)
Velocity distribution or \(\frac{\left(1-{\varepsilon }_{\mathrm{p}}\right){K}_{\mathrm{c}}}{{\varepsilon }_{\mathrm{p}}}\) in Fig. 11
Defined by Eq. 21
Void fraction
\(\frac{{D}_{\mathrm{c}}L}{{r}_{\mathrm{c}}^{2}{v}_{\mathrm{in}}}\)
Collision integral
Pore or viscosity
Collision diameter
Barrier or blank response
Column, crystal or micropore
Outer column
Corrected response
Inner column
Effective parallel Knudsen and surface contribution
Experimental
Index or component
Initial state of column
Inlet or initial
At zero flow
Outlet or outer
Surface or solid
True response
Effective value in the axial direction
Initial or limiting
Back-pressure regulator
Breakthrough curve
Carbon molecular sieve
Constant volume (volumetry)
- Dynamic column breakthrough
Engelhard titanosilicate
Linear driving force
Mass flow controller
Mass flow meter
Metal–organic framework
Mass spectrometer
Needle valve
Point-by-point correction
Pressure indicator
Pressure swing adsorption
Standard liters per minute
Temperature indicator
Tank in series correction
Zero-length column
Ahn, H., Brandani, S.: Dynamics of carbon dioxide breakthrough in a carbon monolith over a wide concentration range. Adsorption 11 , 473–477 (2005)
Article Google Scholar
Amanullah, M., Viswanathan, S., Farooq, S.: Equilibrium, kinetics, and column dynamics of methyl ethyl ketone biodegradation. Ind. Eng. Chem. Res. 39 , 3387–3396 (2000). https://doi.org/10.1021/ie000265m
Article CAS Google Scholar
American Industrial Hygiene Association: ANSI/AIHA Z9. 5-2003 American National Standard Laboratory Ventilation. AIHA, Fairfax (2003)
Google Scholar
Basmadjian, D., Coroyannakis, P., Karayannopoulos, C.: Equilibrium theory revisited. Isothermal fixed-bed sorption of binary systems-II. Non-Langmuir solutes with type I parent isotherms: azeotropic systems. Chem. Eng. Sci. 42 , 1737–1752 (1987). https://doi.org/10.1016/0009-2509(87)80179-3
Bird, R., Stewart, W.E., Lightfoot, E.N.: Transport Phenomena. Wiley, New York (1964)
Bischoff, K.B.: A note on gas dispersion in packed beds. Chem. Eng. Sci. 24 , 607 (1969). https://doi.org/10.1016/0009-2509(69)85030-X
Brandani, F., Rouse, A., Brandani, S., Ruthven, D.M.: Adsorption kinetics and dynamic behavior of a carbon monolith. Adsorption 10 , 99–109 (2004). https://doi.org/10.1023/B:ADSO.0000039866.37214.6a
Brandani, S.: On the chromatographic measurement of equilibrium isotherms using large concentration steps. Adsorption 11 , 231–235 (2005)
Brosillon, S., Manero, M.H., Foussard, J.N.: Mass transfer in VOC adsorption on zeolite: experimental and theoretical breakthrough, curves. Environ. Sci. Technol. 35 , 3571–3575 (2001). https://doi.org/10.1021/es010017x
Article CAS PubMed Google Scholar
Casas, N., Schell, J., Blom, R., Mazzotti, M.: MOF and UiO-67/MCM-41 adsorbents for pre-combustion CO 2 capture by PSA: breakthrough experiments and process design. Sep. Purif. Technol. 112 , 34–48 (2013). https://doi.org/10.1016/j.seppur.2013.03.042
Casas, N., Schell, J., Pini, R., Mazzotti, M.: Fixed bed adsorption of CO 2 /H 2 mixtures on activated carbon: experiments and modeling. Adsorption 18 , 143–161 (2012). https://doi.org/10.1007/s10450-012-9389-z
Cen, P.L., Yang, R.T.: Analytic solution for adsorber breakthrough curves with bidisperse sorbents (zeolites). AIChE J. 32 , 1635–1641 (1986). https://doi.org/10.1002/aic.690321007
Chauveau, R., Grévillot, G., Marsteau, S., Vallières, C.: Values of the mass transfer coefficient of the linear driving force model for VOC adsorption on activated carbons. Chem. Eng. Res. Des. 91 , 955–962 (2013). https://doi.org/10.1016/j.cherd.2012.09.019
Couck, S., Lefevere, J., Mullens, S., Protasova, L., Meynen, V., Desmet, G., Baron, G.V., Denayer, J.F.M.: CO 2 , CH 4 and N 2 separation with a 3DFD-printed ZSM-5 monolith. Chem. Eng. J. 308 , 719–726 (2017). https://doi.org/10.1016/j.cej.2016.09.046
Doong, S.J., Yang, R.T.: Bulk separation of multicomponent gas mixtures by pressure swing adsorption: pore/surface diffusion and equilibrium models. AIChE J. 32 , 397–410 (1986). https://doi.org/10.1002/aic.690320306
Edwards, M.F., Richardson, J.F.: The correlation of axial dispersion data. Can. J. Chem. Eng. 48 , 466–467 (1970). https://doi.org/10.1002/cjce.5450480421
Effendy, S., Xu, C., Farooq, S.: Optimization of a pressure swing adsorption process for nitrogen rejection from natural gas. Ind. Eng. Chem. Res. 56 (18), 5417–5431 (2017)
Farooq, S., Qinglin, H., Karimi, I.A.: Identification of transport mechanism in adsorbent micropores from column dynamics. Ind. Eng. Chem. Res. 41 , 1098–1106 (2002). https://doi.org/10.1021/ie0104621
Farooq, S., Rathor, M.N., Hidajat, K.: A predictive model for a kinetically controlled pressure swing adsorption separation process. Chem. Eng. Sci. 48 , 4129–4141 (1993). https://doi.org/10.1016/0009-2509(93)80259-S
Farooq, S., Ruthven, D.M.: Heat effects in adsorption column dynamics. 2. Experimental validation of the one-dimensional model. Ind. Eng. Chem. Res. 29 , 1084–1090 (1990). https://doi.org/10.1021/ie00102a020
Farooq, S., Ruthven, D.M.: Numerical simulation of a kinetically controlled pressure swing adsorption bulk separation process based on a diffusion model. Chem. Eng. Sci. 46 , 2213–2224 (1991). https://doi.org/10.1016/0009-2509(91)85121-D
Farooq, S., Thaeron, C., Ruthven, D.M.: Numerical simulation of a parallel-passage piston-driven PSA unit. Sep. Purif. Technol. 13 , 181–193 (1998). https://doi.org/10.1016/S1383-5866(98)00042-2
Fournel, L., Mocho, P., Brown, R., Le Cloirec, P.: Modeling breakthrough curves of volatile organic compounds on activated carbon fibers. Adsorption 16 , 147–153 (2010). https://doi.org/10.1007/s10450-010-9207-4
Glueckauf, E., Coates, J.I.: Theory of chromatography. Part IV. The influence of incomplete equilibrium on the front boundary of chromatograms and on the effectiveness of separation. J. Chem. Soc. (1947). https://doi.org/10.1039/jr9470001315
Article PubMed Google Scholar
Goyal, P., Purdue, M.J., Farooq, S.: Adsorption and diffusion of N 2 and CO 2 and their mixture on silica gel. Ind. Eng. Chem. Res. 58 , 19611–19622 (2019). https://doi.org/10.1021/acs.iecr.9b02685
Grande, C.A., Rodrigues, A.E.: Adsorption kinetics of propane and propylene in zeolite 4A. Chem. Eng. Res. Des. 82 , 1604–1612 (2004). https://doi.org/10.1205/cerd.82.12.1604.58029
Grande, C.A., Silva, V.M.T.M., Gigola, C., Rodrigues, A.E.: Adsorption of propane and propylene onto carbon molecular sieve. Carbon NY 41 , 2533–2545 (2003). https://doi.org/10.1016/S0008-6223(03)00304-X
Guiochon, G., Fellinger, A., Golshan-Shirazi, S.: Fundamentals of Preparative and Nonlinear Chromatography. Academic, Boston (2006)
Haghpanah, R., Majumder, A., Nilam, R., Rajendran, A., Farooq, S., Karimi, I.A., Amanullah, M.: Multiobjective optimization of a four-step adsorption process for postcombustion CO 2 capture via finite volume simulation. Ind. Eng. Chem. Res. 52 , 4249–4265 (2013). https://doi.org/10.1021/ie302658y
Han, R., Walton, K.S., Sholl, D.S.: Does chemical engineering research have a reproducibility problem? Annu. Rev. Chem. Biomol. Eng. 10 , 43–57 (2019). https://doi.org/10.1146/annurev-chembioeng-060718-030323
Haq, N., Ruthven, D.M.: Chromatographic study of sorption and diffusion in 4A zeolite. J. Colloid Interface Sci. 112 , 154–163 (1986). https://doi.org/10.1016/0021-9797(86)90077-9
Hassan, M.M., Raghavan, N.S., Ruthven, D.M., Boniface, H.A.: Pressure swing adsorption. Part II: experimental study of a nonlinear trace component isothermal system. AIChE J. 31 , 2008–2016 (1985). https://doi.org/10.1002/aic.690311210
Haynes, H.W., Sarma, P.N.: A model for the application of gas chromatography to measurements of diffusion in bidisperse structured catalysts. AIChE J. 19 , 1043–1046 (1973). https://doi.org/10.1002/aic.690190526
Hefti, M., Joss, L., Marx, D., Mazzotti, M.: An experimental and modeling study of the adsorption equilibrium and dynamics of water vapor on activated carbon. Ind. Eng. Chem. Res. 54 , 12165–12176 (2015). https://doi.org/10.1021/acs.iecr.5b03445
Holman, J.P., Lloyd, J.: Heat Transfer, 10th edn. McGraw-Hill, New York (2008)
Hosseinzadeh Hejazi, S.A.: High-purity oxygen production using Ag-ETS silver-exchanged titanosilicates. In: AIChE Annual Meeting (2017)
Hu, Z., Wang, Y., Farooq, S., Zhao, D.: A highly stable metal–organic framework with optimum aperture size for CO 2 capture. AIChE J. 63 , 4103–4114 (2017). https://doi.org/10.1002/aic.15837
Huang, C.C., Fair, J.R.: Study of the adsorption and desorption of multiple adsorbates in a fixed bed. AIChE J. 34 , 1861–1877 (1988). https://doi.org/10.1002/aic.690341112
Hwang, K.S., Lee, W.K.: The adsorption and desorption breakthrough behavior of carbon monoxide and carbon dioxide on activated carbon. Effect of total pressure and pressure-dependent mass transfer coefficients. Sep. Sci. Technol. 29 , 1857–1891 (1994). https://doi.org/10.1080/01496399408002177
Joss, L., Mazzotti, M.: Modeling the extra-column volume in a small column setup for bulk gas adsorption. Adsorption 18 , 381–393 (2012)
Kapoor, A., Yang, R.T.: Roll-up in fixed-bed, multicomponent adsorption under pore-diffusion limitation. AIChE J. 33 , 1215–1217 (1987). https://doi.org/10.1002/aic.690330717
Kärger, J., Ruthven, D.M., Theodorou, D.N.: Diffusion in Nanoporous Materials. Wiley, Weinheim (2012)
Book Google Scholar
Khurana, M., Farooq, S.: Adsorbent screening for postcombustion CO 2 capture: a method relating equilibrium isotherm characteristics to an optimum vacuum swing adsorption process performance. Ind. Eng. Chem. Res. 55 , 2447–2460 (2016). https://doi.org/10.1021/acs.iecr.5b04531
Kim, Y.H., Kim, J.J., Lee, C.H.: Adsorptive cyclic purification process for CO 2 mixtures captured from coal power plants. AIChE J. 63 , 1051–1063 (2017). https://doi.org/10.1002/aic.15440
Knox, J.C., Ebner, A.D., Levan, M.D., Coker, R.F., Ritter, J.A.: Limitations of breakthrough curve analysis in fixed-bed adsorption. Ind. Eng. Chem. Res. 55 , 4734–4748 (2016). https://doi.org/10.1021/acs.iecr.6b00516
Article CAS PubMed PubMed Central Google Scholar
Langer, G., Roethe, A., Roethe, K.P., Gelbin, D.: Heat and mass transfer in packed beds-III. Axial mass dispersion. Int. J. Heat Mass Transf. 21 , 751–759 (1978). https://doi.org/10.1016/0017-9310(78)90037-6
Leperi, K.T., Chung, Y.G., You, F., Snurr, R.Q.: Development of a general evaluation metric for rapid screening of adsorbent materials for postcombustion CO 2 capture. ACS Sustain. Chem. Eng. 7 , 11529–11539 (2019). https://doi.org/10.1021/acssuschemeng.9b01418
Leva, M., Grummer, M.: Heat transfer to gases through packed tubes. Ind. Eng. Chem. 40 , 415–419 (1948). https://doi.org/10.1021/ie50459a012
Malek, A., Farooq, S.: Determination of equilibrium isotherms using dynamic column breakthrough and constant flow equilibrium desorption. J. Chem. Eng. Data (1996). https://doi.org/10.1021/je950178e
Malek, A., Farooq, S.: Kinetics of hydrocarbon adsorption on activated carbon and silica gel. AIChE J. 43 , 761–776 (1997). https://doi.org/10.1002/aic.690430321
Malek, A., Farooq, S., Rathor, M.N., Hidajat, K.: Effect of velocity variation due to adsorption–desorption on equilibrium data from breakthrough experiments. Chem. Eng. Sci. 50 , 737–740 (1995). https://doi.org/10.1016/0009-2509(94)00245-M
Murillo, R., García, T., Aylón, E., Callén, M.S., Navarro, M.V., López, J.M., Mastral, A.M.: Adsorption of phenanthrene on activated carbons: breakthrough curve modeling. Carbon NY 42 , 2009–2017 (2004). https://doi.org/10.1016/j.carbon.2004.04.001
Najafi Nobar, S., Farooq, S.: Experimental and modeling study of adsorption and diffusion of gases in Cu-BTC. Chem. Eng. Sci. 84 , 801–813 (2012). https://doi.org/10.1016/j.ces.2012.05.022
Nitta, T., Kuro-Oka, M., Katayama, T.: An adsorption isotherm of multi-site occupancy model for heterogeneous surface. J. Chem. Eng. Jpn 17 , 45–52 (1984). https://doi.org/10.1252/jcej.17.45
Park, J., Rubiera Landa, H.O., Kawajiri, Y., Realff, M.J., Lively, R.P., Sholl, D.S.: How well do approximate models of adsorption-based CO 2 capture processes predict results of detailed process models? Ind. Eng. Chem. Res. (2020). https://doi.org/10.1021/acs.iecr.9b05363
Peter, S.A., Baron, G.V., Gascon, J., Kapteijn, F., Denayer, J.F.M.: Dynamic desorption of CO 2 and CH 4 from amino-MIL-53(Al) adsorbent. Adsorption 19 , 1235–1244 (2013). https://doi.org/10.1007/s10450-013-9564-x
Rajagopalan, A.K., Avila, A.M., Rajendran, A.: Do adsorbent screening metrics predict process performance? A process optimisation based study for post-combustion capture of CO 2 . Int. J. Greenh. Gas Control 46 , 76–85 (2016). https://doi.org/10.1016/j.ijggc.2015.12.033
Rajendran, A., Kariwala, V., Farooq, S.: Correction procedures for extra-column effects in dynamic column breakthrough experiments. Chem. Eng. Sci. 63 , 2696–2706 (2008). https://doi.org/10.1016/j.ces.2008.02.023
Rezaei, F., Webley, P.: Structured adsorbents in gas separation processes. Sep. Purif. Technol. 70 (3), 243–256 (2010)
Rhee, H.K., Aris, R., Amundson, N.R.: First-Order Partial Differential Equations. Dover Publications, Mineola (2001)
Ribeiro, A.M., Sauer, T.P., Grande, C.A., Moreira, R.F.P.M., Loureiro, J.M., Rodrigues, A.E.: Adsorption equilibrium and kinetics of water vapor on different adsorbents. Ind. Eng. Chem. Res. 47 , 7019–7026 (2008). https://doi.org/10.1021/ie701732x
Ritter, J.A., Bhadra, S.J., Ebner, A.D.: On the use of the dual-process Langmuir model for correlating unary equilibria and predicting mixed-gas adsorption equilibria. Langmuir 27 , 4700–4712 (2011). https://doi.org/10.1021/la104965w
Ruthven, D.M.: Principles of Adsorption and Adsorption Processes. Wiley, New York (1984)
Ruthven, D.M., Farooq, S., Knaebel, K.: Pressure Swing Adsorption. VCH Publishers, New York (1994)
Ruthven, D.M., Thaeron, C.: Performance of a parallel passage adsorbent contactor. Sep. Purif. Technol. 12 , 43–60 (1997). https://doi.org/10.1016/S1383-5866(97)00016-6
Saleman, T.L.H., Watson, G.C.Y., Rufford, T.E., Hofman, P.S., Chan, K.I., May, E.F.: Capacity and kinetic measurements of methane and nitrogen adsorption on H+-mordenite at 243–303 K and pressures to 900 kPa using a dynamic column breakthrough apparatus. Adsorption 19 , 1165–1180 (2013). https://doi.org/10.1007/s10450-013-9546-z
Seidel-Morgenstern, A.: Experimental determination of single solute and competitive adsorption isotherms. J. Chromatogr. A 1037 , 255–272 (2004). https://doi.org/10.1016/j.chroma.2003.11.108
Sharma, I., Mennitto, R., Friedrich, D., Brandani, S.: Combining the nonuniform structure and flow maldistribution for the accurate prediction of the process performance of monolithic adsorbent systems. Ind. Eng. Chem. Res. 59 , 3162–3172 (2020). https://doi.org/10.1021/acs.iecr.9b05845
Son, K.N., Weibel, J.A., Knox, J.C., Garimella, S.V.: Limitations of the axially dispersed plug-flow model in predicting breakthrough in confined geometries. Ind. Eng. Chem. Res. 58 , 3853–3866 (2019). https://doi.org/10.1021/acs.iecr.8b05925
Thommes, M., Kaneko, K., Neimark, A.V., Olivier, J.P., Rodriguez-Reinoso, F., Rouquerol, J., Sing, K.S.W.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87 , 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
Todd, R.S., He, J., Webley, P.A., Beh, C., Wilson, S., Lloyd, M.A.: Fast finite-volume method for PSA/VSA cycle simulation—experimental validation. Ind. Eng. Chem. Res. 40 , 3217–3224 (2001). https://doi.org/10.1021/ie0008070
Wakao, N., Kaguei, S., Funazkri, T.: Effect of fluid dispersion coefficients on particle-to-fluid heat transfer coefficients in packed beds Correlation of Nusselt numbers. Chem. Eng. Sci. 34 , 325–336 (1979). https://doi.org/10.1016/0009-2509(79)85064-2
Walton, K.S., LeVan, M.D.: Effect of energy balance approximations on simulation of fixed-bed adsorption. Ind. Eng. Chem. Res. 44 , 7474–7480 (2005). https://doi.org/10.1021/ie050065g
Wankat, P.C.: Large-Scale Adsorption and Chromatography. CRC Press, Boca Raton (1986)
Webley, P.A., He, J.: Fast solution-adaptive finite volume method for PSA/VSA cycle simulation; 1 single step simulation. Comput. Chem. Eng. 23 , 1701–1712 (2000). https://doi.org/10.1016/S0098-1354(99)00320-8
Wicke, E.: Bedeutung der molekularen Diffusion für chromatographische Verfahren. Ber. Bunsenges. Phys. Chem. 77 , 160–171 (1973). https://doi.org/10.1002/bbpc.19730770305
Wilkins, N.S., Rajendran, A.: Measurement of competitive CO 2 and N 2 adsorption on Zeolite 13X for post-combustion CO 2 capture. Adsorption 25 , 115–133 (2019). https://doi.org/10.1007/s10450-018-00004-2
Wilkins, N.S., Sawada, J.A., Rajendran, A.: Measurement of competitive CO 2 and H 2 O adsorption on zeolite 13X for post-combustion CO 2 capture. Adsorption (2020). https://doi.org/10.1007/s10450-020-00199-3
Wu, C.W., Vemula, R.R., Kothare, M.V., Sircar, S.: Experimental study of a novel rapid pressure-swing adsorption based medical oxygen concentrator: effect of the adsorbent selectivity of N 2 over O 2 . Ind. Eng. Chem. Res. 55 , 4676–4681 (2016). https://doi.org/10.1021/acs.iecr.5b04570
Xiao, P., Zhang, J., Webley, P., Li, G., Singh, R., Todd, R.: Capture of CO 2 from flue gas streams with zeolite 13X by vacuum-pressure swing adsorption. Adsorption 14 , 575–582 (2008). https://doi.org/10.1007/s10450-008-9128-7
Yagi, S., Kunii, D.: Studies on effective thermal conductivities in packed beds. AIChE J. 3 , 373–381 (1957). https://doi.org/10.1002/aic.690030317
Yang, R.T.: Gas Separation by Adsorption Processes. Imperial College Press, London (2013)
Download references
Author information
Authors and affiliations.
Department of Chemical and Materials Engineering, Donadeo Innovation Centre of Engineering, University of Alberta, 9211-116 Street NW, Edmonton, AB, T6G 1H9, Canada
Nicholas Stiles Wilkins & Arvind Rajendran
Department of Chemical & Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore, 117585, Singapore
Shamsuzzaman Farooq
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Shamsuzzaman Farooq .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 1292 kb)
Rights and permissions.
Reprints and permissions
About this article
Wilkins, N.S., Rajendran, A. & Farooq, S. Dynamic column breakthrough experiments for measurement of adsorption equilibrium and kinetics. Adsorption 27 , 397–422 (2021). https://doi.org/10.1007/s10450-020-00269-6
Download citation
Received : 04 June 2020
Revised : 02 September 2020
Accepted : 15 September 2020
Published : 30 September 2020
Issue Date : April 2021
DOI : https://doi.org/10.1007/s10450-020-00269-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Equilibrium
- Experimental protocol
- Data presentation and analysis
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List

Effects of Structure and Composition of Adsorbents on Competitive Adsorption of Gaseous Emissions: Experiment and Modeling
Adam verner, jonáš tokarský, tomáš najser, lenka matějová, kateřina mamulová kutláková, václav peer.
- Author information
- Article notes
- Copyright and License information
Correspondence: [email protected]
Received 2023 Jan 31; Revised 2023 Feb 9; Accepted 2023 Feb 11; Collection date 2023 Feb.
Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( https://creativecommons.org/licenses/by/4.0/ ).
Dangerous gases arising from combustion processes must be removed from the air simply and cheaply, e.g., by adsorption. This work is focused on competitive adsorption experiments and force field-based molecular modeling of the interactions at the molecular level. Emission gas, containing CO, NO, SO 2 , and CO 2 , was adsorbed on activated carbon, clay mineral, silicon dioxide, cellulose, or polypropylene at two different temperatures. At 20 °C, activated carbon had the highest NO and SO 2 adsorption capacity (120.83 and 3549.61 μg/g, respectively). At 110 °C, the highest NO and SO 2 adsorption capacity (6.20 and 1182.46 μg/g, respectively) was observed for clay. CO was adsorbed very weakly, CO 2 not at all. SO 2 was adsorbed better than NO, which correlated with modeling results showing positive influence of carboxyl and hydroxyl functional groups on the adsorption. In addition to the wide range of adsorbents, the main novelty of this study is the modeling strategy enabling the simulation of surfaces with pores of controllable sizes and shapes, and the agreement of the results achieved by this strategy with the results obtained by more computationally demanding methods. Moreover, the agreement with experimental data shows the modeling strategy to be a valuable tool for further adsorption studies.
Keywords: gaseous emission, competitive adsorption, molecular modeling, morphology, adsorbent structure
1. Introduction
In recent years, more than ever before, the importance of monitoring the evolution of the amount of pollutants in the environment has increased [ 1 ]. People and nature in general need optimal conditions for their efficient functioning, which is related to the reduction of destructive factors and the increase of constructive factors in the environment. Manufacturing industry is accompanied by the disruption of natural resources during the acquisition of input materials, a number of chemical reactions or energy processes during production, and last but not least, the disposal of waste, i.e., unused or unusable products. The main sources of pollution include the manufacturing and processing industry [ 2 ] as well as the energy industry [ 3 ] and transportation [ 4 ], with which the increasing production of specifically air pollutants is connected.
Despite the fact that thermochemical conversion is beginning to be replaced by the use of renewable resources worldwide, the combustion process of various materials (coal, gas, heating oils, wood, biomass, etc.) still contributes significantly to air pollution [ 5 , 6 ]. Even with the effective use of plant waste [ 7 , 8 ], it is still challenging to monitor and reduce the risks related to the production of arising pollutants. While solid pollutants consist primarily of particulate matter of various grain sizes and NH 3 , gaseous pollutants mainly include CO 2 , CO, SO 2 , nitrogen oxides (NO x ) [ 9 , 10 ], volatile organic compounds and polycyclic aromatic hydrocarbons [ 6 , 10 ]. Solid particles released during the combustion process can be easily captured by various types of solid or liquid filter systems. However, the removal of emission gases from the air is generally more complex, due to the gaseous state of the pollutants, the required chemical selectivity, etc. The most studied is CO 2 removal from the air [ 11 ]. Absorption of CO 2 in gas-liquid systems with methanol, poly-(ethylene glycol) dimethyl ether [ 12 ] or amine solutions [ 13 ] as absorbing phases was investigated. However, adsorption methods in gas-solid systems, i.e., weaker physisorption or stronger chemisorption [ 11 , 14 , 15 ], are used more often. The most effective adsorption materials for CO 2 include activated carbons [ 16 , 17 ], zeolites [ 18 ], porous oxides (e.g., calcium oxides [ 19 ] or silica gel [ 20 ]), hydrotalcites [ 21 ], amines [ 22 ] or metal-organic frameworks [ 11 , 23 , 24 ].
On the other hand, the adsorption mechanisms and suitable adsorbents of NO x , CO, and SO 2 have been considerably less investigated. Research on CO and NO adsorption using Pd catalysts [ 25 ], possibly Au alloys [ 26 ] or various modifications of activated carbon [ 27 ] should be mentioned. In the case of SO 2 and NO, adsorption mechanisms on modified activated carbon [ 27 , 28 ] and bimetallic surfaces containing noble and transition metals [ 29 ] were investigated. Zeolites are also efficient adsorbents of CO 2 , SO 2 , and NO [ 30 ].
Research works mostly deal with specific adsorption materials for a specific gas, but materials in various forms sorbing several types of emission gases at the same time by the mechanism of competitive adsorption have not yet been sufficiently investigated. There is also a lack of research on interactions at the molecular level during the competitive adsorption of emission gases. Therefore, in this work, the process of competitive adsorption of emission gases on different materials with different physical and chemical properties was studied both experimentally and via force field-based molecular modeling. Activated carbon is extensively researched and modeled in connection with adsorption [ 31 , 32 , 33 , 34 ]. Therefore, it was used for comparing the obtained data and verifying the modeling strategy. Adsorbent materials whose potential in the field of competitive adsorption of emission gases has not yet been established were used, and adsorption capacities were determined. Unlike DTF modeling methods that are usually used, the modeling strategy introduced here enabled the creation of larger models with pores of precisely controllable sizes and shapes. The influence of chemical and structural factors on competitive adsorption was clarified, which can be used further in research on other materials or as a prediction tool of adsorption.

2. Materials and Methods
2.1. materials.
Adsorption experiments were carried out using a special emission gas (EG) having concentrations of emission components similar to the real concentrations produced during the combustion of various alternative fuels, such as biomass, sewage sludge, etc. [ 35 ]. The EG consisted of 197.3 vol. ppm NO (199.9 w / w ppm), 197.3 vol. ppm SO 2 (426.8 w / w ppm), 395 vol. ppm CO (373.6 w / w ppm), and 9.96 vol. % CO 2 (14.80 w / w %) in nitrogen (SIAD Czech s.r.o., Ostrava, Czech Republic). In addition, the nitrogen was also used to purge the adsorption path.
The following materials commonly used in hazardous chemical spill incidents were used as adsorbents in this study: activated carbon (AC; MA C6 D40 CZ, Resorbent, s.r.o., Ostrava, Czech Republic), clay (CL; Bentonite Puranit, LIPERA s.r.o., Velke Bilovice, Czech Republic), and (from REO AMOS s.r.o., Ostrava, Czech Republic) silicon dioxide (SiO 2 ), cellulose (CEL) and polypropylene (PP).
2.2. Characterization of Samples
A photoelectron spectrometer with a hemispherical VG SCIENTA R3000 analyzer (Prevac, Rogów, Poland) was used for XPS analysis of AC. The photoelectron spectra were recorded using a monochromatized aluminum Al Kα source ( E = 1486.6 eV) and a low-energy electron flood gun (FS40A-PS). The base pressure in the analysis chamber was 5·10 −9 mbar. Spectra were recorded with a constant pass energy of 100 eV for the survey and high-resolution spectra. The binding energy scale was calibrated using the Au 4f7/2 line of a cleaned gold sample at 84.0 eV. The composition and chemical surrounding of the sample surface were investigated on the basis of the areas and binding energies of O 1s and C 1s photoelectron peaks. The fitting of high-resolution spectra was performed using CasaXPS software.
The moisture content of the samples was determined by Kern ABT 320-4NM (Kern & Sohn GmbH, Balingen, Germany). Particle density was determined by measuring the volume of the sample in water and the weight with MA 110.R laboratory scale (RADWAG Váhy s.r.o., Šumperk, Czech Republic). Atmospheric pressure and pressure in the adsorption system were measured with GPB 3300 (GHM-Greisinger s.r.o., Regenstauf, Germany) and Kimo MP 210 (Kimo Instruments, Mumbai, India), respectively. The concentration of NO, SO 2 and CO was measured by the infrared spectrometer PG-350 (Horiba, Palaiseau, France). Nitrogen physisorption at 77 K was measured using 3Flex apparatus (Micromeritics, Norcross, CA, USA). Prior to physisorption analysis, each material was degassed under pressure of 0.6 bar and temperature of 350 °C. This step was done for all materials to release physisorbed moisture. After such pre-treatment, the nitrogen adsorption–desorption isotherms at 77 K of all materials were measured for the relative pressure range p / p 0 ~1 × 10 −7 –0.99. Nitrogen adsorption–desorption data were processed using BET theory, the t-plot method and the BJH method applied on the adsorption branch of the nitrogen adsorption–desorption isotherm, by using either the Carbon Black STSA standard isotherm (for carbonaceous materials) or Broekhoff-De Boer standard isotherm (for inorganic materials). The slit or cylindrical-pore geometry of mesopores and macropores characterized by pore width or diameter, respectively, were assumed. From measured data, it was possible to reliably obtain the specific surface area, S BET (m 2 /g), the mesopore–macropore surface area, S meso (m 2 /g), the micropore volume, V micro (mmliq 3 /g) and mesopore–macropore size distribution characterized by the pore width or diameter. The net pore volume, V net (mm 3 liq/g), was evaluated from the adsorbed amount of nitrogen at p / p 0 ~0.99. The micropore size distribution was evaluated from the adsorbed amount of nitrogen at p / p 0 ~1 × 10 −7 –0.05 using the Horwath–Kawazoe solution and assuming the slit-pore geometry for both carbonaceous and inorganic materials.
X-ray powder diffraction (XRPD) patterns were recorded under Co Kα irradiation ( λ = 0.1789 nm, U = 35 kV, I = 25 mA) using the Bruker D8 Advance diffractometer (Bruker AXS, Karlsruhe, Germany) equipped with a fast position sensitive detector VÅNTEC 1. Measurements were carried out in the reflection mode, powder samples were pressed in a rotational holder, and a goniometer was used with the Bragg–Brentano geometry in 2θ range from 3 to 80°, step size 0.03°. The phase composition was evaluated using database PDF 2 Release 2020 (International Centre for Diffraction Data).
2.3. Sample Preparation
The powder samples (AC, CL, SiO 2 ) were crushed and sieved into three granulometric fractions—0.16–0.315 mm, 0.315–0.63 mm, 0.63–1.00 mm ( Table 1 )—to ensure laminar flow in the adsorption chamber (Ad) whose inner tube diameter was 10 mm. CEL and PP were used in their original form, i.e., pellets and nonwoven, respectively. Each type of sample was used in its wet and dried state. The samples were dried in a drying oven Binder FP 400.
Basic information about the adsorbing materials used, such as particle density and material humidity, and its forms and labeling.
AC—activated carbon, CL—clay, SiO 2 —silicon dioxide, CEL—cellulose, PP—polypropylene.
Particle density and humidity of the adsorbents were determined, and the samples were labeled according to the granulometric fraction ( Table 1 ).
2.4. Adsorption System
The adsorption experiments took place in a closed system consisting of PTFE hoses, stainless steel fittings, the stainless steel Ad and analytical instruments ( Figure 1 ). The Ad was a stainless steel tube with an inner diameter of 10 mm. A volume of 3 cm 3 was calculated from the volume of the catalyst V cat according to the formula:
where Q V is volumetric gas flow rate per hour (0.5 L/min = 30,000 cm 3 /h) and GHSV is gas hourly space velocity (10,000 h −1 ) [ 36 ].
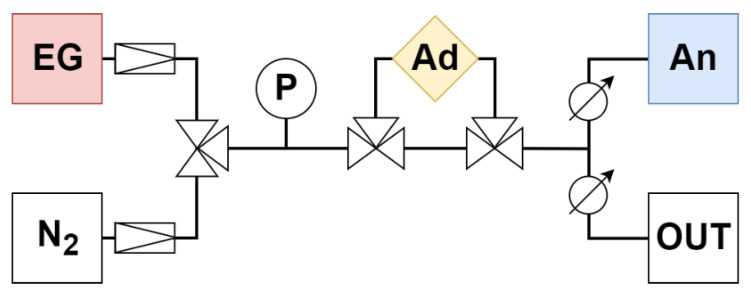
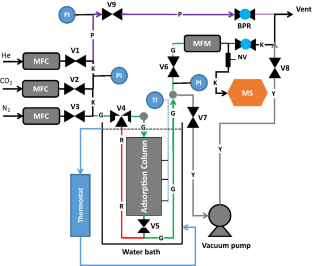
Diagram of adsorption apparatus with fittings. EG—emission gas, N 2 —nitrogen, P—pressure gauge, Ad—adsorption chamber, An—analyzer, OUT—overflow branch.
The gas traveled from the gas cylinders (EG—emission gas, N 2 —nitrogen) through the pressure gauge (P) and the adsorbent (Ad) to the analyzer (An) at a speed of 0.5 L/min (analyzer parameter). The rest was discharged through the overflow branch (OUT) ( Figure 1 ).
First, the entire adsorption apparatus was filled with nitrogen (including the Ad branch). Subsequently, the source gas was switched from N 2 to EG through the branch without Ad. The measurement of the concentration of emission components with the analyzer was switched on. As soon as the concentration values stabilized, thereby determining the actual concentrations of gaseous components in EG, the gas path was switched to the Ad branch, where adsorption took place. The measurement was terminated after the initial concentration was again reached (the actual concentration in the EG bottle). The measurement took place at 20 °C (with wet adsorbents) and 110 °C (with dried adsorbents and Ad in the drying oven Binder FP 400 (Binder GMBh, Tuttlingen, Germany). Each sample was measured three times and the results were averaged.
The total amount of each adsorbed gaseous component was obtained by integrating the area between the adsorption curve and the original concentration value of the given emission component, and using the ideal gas equation (where the amount of substance n is expressed as m / M ) for expressed mass m of the adsorbed gas component:
where p (Pa) is pressure in the adsorption system, V (dm 3 ) is volume of the adsorbent, M (g·mol −1 ) is molar mass of the gaseous component, R (J·K −1 ·mol −1 ) is the gas constant, and T (K) is temperature in the adsorption system [ 37 ].
Adsorbed mass m was related to one gram of wet or dried adsorbent expressed in μg/g.
2.5. Atomistic Models and Modeling Strategy
Building of the initial models of EG molecule and adsorbent surface, geometry optimizations, molecular dynamics, and energy calculations were carried out in Biovia Materials Studio 7.0 (MS) modeling environment [ 38 ]. Triclinic unit cells of CEL (a = 8.549 Å, b = 9.568 Å, c = 10.000 Å, α = 102.10°, β = 106.54°, γ = 55.46°), and PP (a = 8.348 Å, b = 13.505 Å, c = 10.000 Å, α = 75.68°, β = 39.54°, γ = 61.01°), both containing two polymer chains, were created according to Chen et al. [ 39 ] and Verenich et al. [ 40 ], respectively ( Figure 2 ).
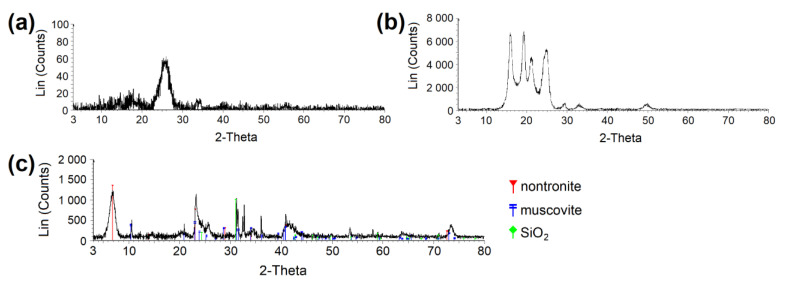
XRPD diffractogram proving the crystalline phase in ( a ) CEL and ( b ) PP. ( c ) Determination of clay phases in clay material. CEL—cellulose, PP—polypropylene.
AC orthorhombic unit cell (a = b = 2.460 Å, c = 6.670 Å, α = β = 90°, γ = 120°) was prepared according to Wyckoff [ 41 ]. SiO 2 trigonal (a = b = 4.913 Å, α = β = 90°, γ = 95.18°) and MUS monoclinic unit cells (a = 5.194 Å, b = 8.996 Å, c = 20.096 Å, α = β = 90°, γ = 120°, layer thickness ~6.7 Å, interlayer distance ~3.3 Å) were imported from the MS database. Substitutions and interlayer cations were added to MUS so that MUS and NON unit cells met the chemical formulas ( K 0.86 N a 0.10 ) ( A l 1.90 F e 0.05 2 + M g 0.06 F e 0.02 3 + T i 0.01 ) ( S i 3.02 A l 0.98 ) O 10 ( O H ) 1.99 F 0.01 and ( C a 1.50 N a 0.5 ) ( F e 1.84 3 + A l 0.15 M g 0.02 □ 0.99 ) ( S i 3.46 A l 0.38 F e 0.16 3 + ) O 10 ( O H ) 2 , respectively, according to Weiss et al. [ 42 ]. Chemical formulas of prepared MUS and NON models were ( K 180 N a 18 ) ( A l 334 F e 9 2 + M g 11 F e 4 3 + T i 2 ) ( S i 540 A l 180 ) O 1800 ( O H ) 358 F 2 and ( C a 270 N a 90 ) ( F e 330 3 + A l 25 M g 5 □ 180 ) ( S i 620 A l 70 F e 30 3 + ) O 1800 ( O H ) 360 , respectively. CEL, PP, SiO 2 , and AC periodic unit cells were enlarged to ~50 × 50 Å with a surface height and additional vacuum slab height of 50 Å and 350 Å, respectively. The CEL, PP, SiO 2 , and AC surfaces were further modified to simulate nanopores with dimensions of (width × height) ~30 × 30 Å ( Figure 3 ) and molecular pores with dimensions of 5.1 × 7.1 Å (CEL), 6.5 × 5.9 Å (PP), 5.6 × 5.7 Å (SiO 2 ), and 5.9 × 6.3 Å (AC) ( Figure 4 ).
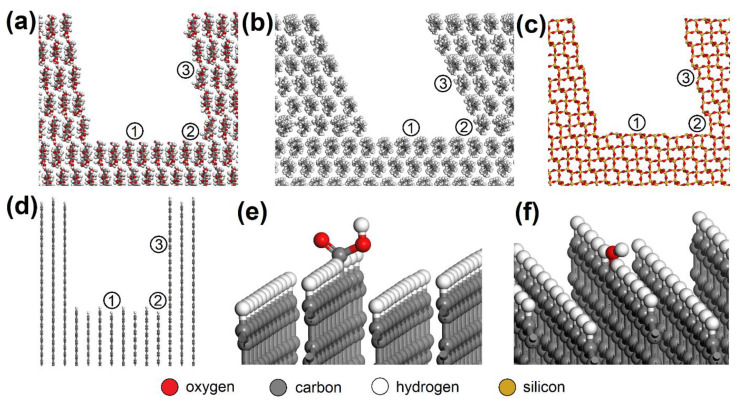
Nanopores in the ( a ) CEL, ( b ) PP, ( c ) SiO 2 , and ( d ) AC surfaces with spots where the NO or SO 2 molecule was placed: (1) bottom, (2) corner, (3) side. NO or SO 2 molecules were also placed on the AC surfaces with -COOH or -OH groups added, e.g. ( e ) bottom_COOH and ( f ) bottom_OH. CEL—cellulose, PP—polypropylene, SiO 2 —silicon dioxide, AC—activated carbon.
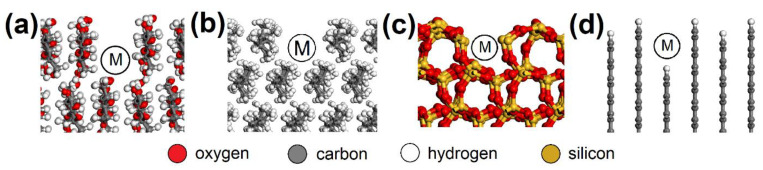
Molecular pores in the ( a ) CEL, ( b ) PP, ( c ) SiO 2 , and ( d ) AC surfaces with spots (M) where the NO or SO 2 molecule was placed. CEL—cellulose, PP—polypropylene, SiO 2 —silicon dioxide, AC—activated carbon.
Due to the XPS analysis of AC adsorbents establishing 3.67 % carboxyl and 4.61 % hydroxyl groups on the surface, models with -COOH (bottom_COOH, corner_COOH, cavity_COOH) and -OH (bottom_OH, corner_OH, cavity_OH) added to the bare AC surfaces (bottom, corner, cavity) were also prepared ( Figure 3 e,f). The main advantage of using this approach to building AC models based on a graphitic structure is the possibility it offers of creating pores and cavities of controllable sizes [ 43 ].
MUS and NON periodic unit cells were enlarged to ~50 × 50 Å with a surface containing two clay layers with two interlayers and a vacuum slab added with a height of 350 Å. NO and SO 2 molecules were placed on various spots of the surfaces ( Figure 5 ). Three models were created for each “EG molecule/adsorbent surface spot” combination.
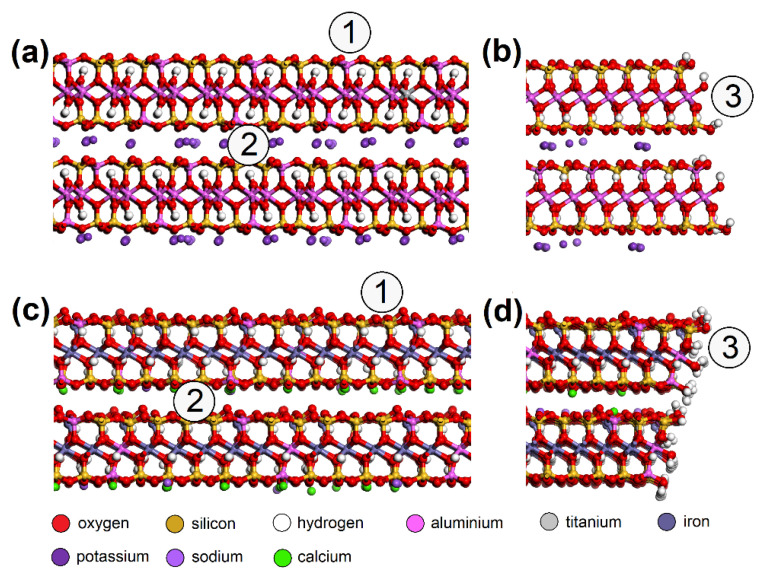
Molecular models of MUS ( a ) surface, ( b ) edge; and NON ( c ) surface and ( d ) edge—with spots where the NO or SO 2 molecule was placed: (1) surface, (2) interlayer, (3) edge. MUS—muscovite, NON—nontronite.
An MS/Forcite module was used for geometry optimization of each model. The COMPASS force field parameterized atoms and assigned their charges [ 44 ], which was verified for CEL [ 45 ], PP [ 46 ], SiO 2 [ 47 ], and AC [ 43 ]. A universal force field, with the QEq module setting charges, was used for models containing MUS and NON [ 48 ].
The Smart algorithm (implemented in MS) with 5·10 5 steps was used. Convergence thresholds for displacement, force, and energy were 5·10 −5 Å, 5·10 −3 kcal·mol −1 ·Å −1 , and 1·10 −4 kcal·mol −1 , respectively. Cell parameters were not optimized. Interaction energy ( E int ; kcal/mol) was calculated for each optimized model from potential energies ( E p ) using the following equation:
where E p 1 is E p of a whole model, E p 2 is E p of a surface, and E p 3 is E p of a NO/SO 2 molecule. The lower the E int value was, the stronger the interaction between NO/SO 2 and the surfaces.
For molecular dynamics, periodic models of AC, MUS, and NON with dimensions of ~17 × 17 Å (α = β = 90°) were prepared. The AC model contained three graphene layers, while the MUS and NON models contained two clay layers with one interlayer. All models had a vacuum slab, 1000 Å high, perpendicular to the surfaces. A total of 28 NO and 28 SO 2 molecules were evenly placed on one side of the surfaces. The MD/Forcite module and COMPASS or Universal force field for AC or MUS/NON models, respectively, were used for molecular dynamics with NVT ensemble, Nosé thermostat, random initial velocities, 293 K temperature, and 5 ns total simulation time.
3. Results and Discussion
3.1. physisorption.
Nitrogen physisorption measurements ( Figure 6 a,c,e; Table 2 ) proved that individual groups of adsorbents differ very much concerning their textural properties. According to the surface area and porosity, the adsorbents used can be ordered as follows (from largest to smallest): AC > CL > SiO 2 > CEL > PP.
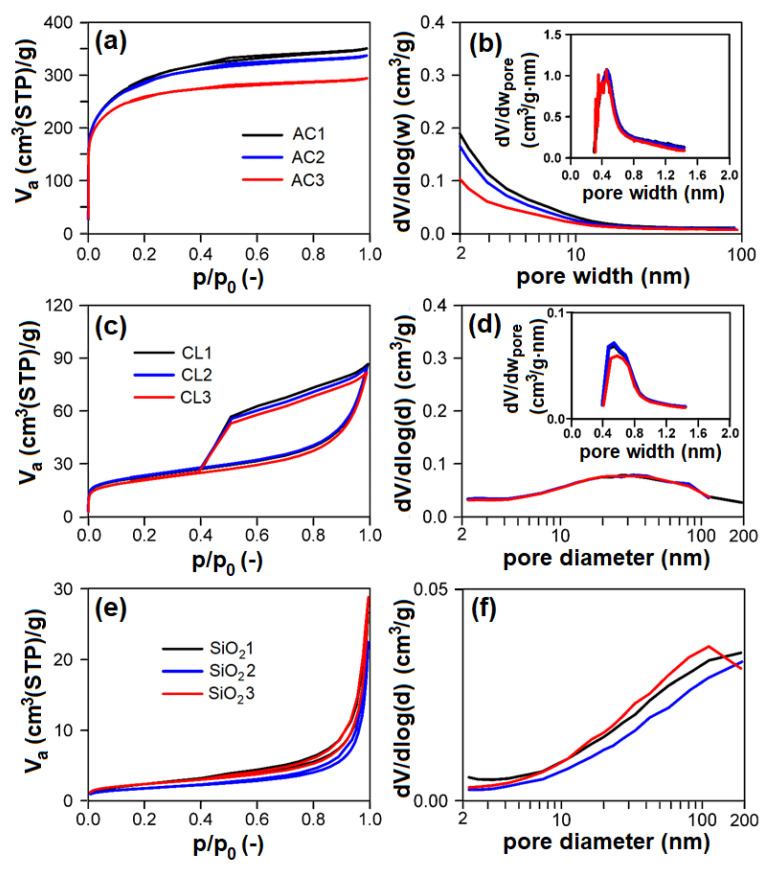
(left: ( a , c , e )) Measured nitrogen adsorption–desorption isotherms at 77 K, (right: ( b , d , f )) evaluated mesopore–macropore-size distributions of adsorbents and (inset) evaluated micropore size distributions of adsorbents: ( a , b ) AC; ( c , d ) CL; ( e , f ) SiO 2 . AC1-3, CL1-3, SiO 2 1-3—activated carbon, clay, and silicon dioxide respectively (1-3—three granulometric fractions; see Table 1 ), V a —adsorbed volume of nitrogen, STP—standard temperature and pressure, p/p 0 —relative pressure, dV/dlog(w) —derivation of adsorbed volume of nitrogen divided by derivation of logarithm of pore width, dV/dw pore —derivation of adsorbed volume of nitrogen divided by derivation of pore width, dV/dlog(d) —derivation of adsorbed volume of nitrogen divided by derivation of logarithm of pore diameter.
Determined textural properties of adsorbents.
* Since adsorbent belongs to meso–macroporous solids, the mesopores surface area S meso equals the specific surface area S BET . AC1-3, CL1-3, SiO 2 1-3—activated carbon, clay, and silicon dioxide, respectively (1-3—three granulometric fractions; see Table 1 ), CEL—cellulose, PP—polypropylene; S BET —specific surface area, S meso —mesopore–macropore surface area, V micro —micropore volume, V net —net pore volume, n. d.—not defined.
AC showed the type I adsorption isotherm according to IUPAC classification [ 49 ], typical for microporous materials ( Figure 6 a). Evaluated textural parameters ( Table 2 ) and pore-size distributions ( Figure 6 b and insets) definitively correspond to this feature. AC showed micropores of 0.48 nm width with a volume of 359–376 mm 3 liq/g, and mesopore surface area in the range of 114–209 m 2 /g. With increasing particle-size fraction in the range of 0.16–1 mm, the mesopore surface area and net pore volume of AC logically decreases with no effect on micropore volume.
CL showed the mixed I + IV type of adsorption isotherm (according to IUPAC classification) typical for mesoporous materials with broad mesopores/macropores and some micropores ( Figure 6 c,d). CL possessed ~14 mm 3 liq/g of micropores of ~0.6 nm width ( Figure 6 d inset) and broad mesopores/macropores of 47–50 m 2 /g surface area ( Figure 6 d). There are no differences between individual particle-size fractions of CL, the properties of individual particle-size fractions being identical.
Concerning SiO 2 , these samples show the type III adsorption isotherm (according to IUPAC classification) with narrow steep hysteresis loop ( Figure 6 e), typical for macroporous/nonporous materials with large macropores, which corresponds to the pore-size distributions shown in Figure 6 f, proving the presence of macropores having diameter >100 nm. Textural properties (i.e., pore surface area, net pore volume) of SiO 2 are comparable; there are no differences among particle-size fractions. PP and CEL belong among nonporous materials with very low surface area.
3.2. Emission Gas Adsorption
With the exception of PP, all adsorbent materials were able to adsorb at least one of the EG components ( Table 3 ). CO 2 was adsorbed on none of the adsorbents. Adsorption of CO on SiO 2 was not observed at any temperature. Granulometry had a significant effect on the adsorbed amount of gases—the smaller the particles, the more adsorbed EG. At the adsorption temperature of 20 °C, AC on average adsorbed the most amount of both NO (9× higher than CL, 20× higher than CEL; Table 3 ) and SO 2 (1.5× higher than CL, 3× higher than CEL, and 54× higher than SiO 2 ; Table 3 ). At the adsorption temperature of 110 °C, all of the adsorbents adsorbed NO in very small amounts in contrast to SO 2 , which was adsorbed significantly more on average, and most on CL (6× higher than on AC, 7× higher than on CEL and 82× higher than on SiO 2 ; Table 3 ). CEL was the only one to adsorb CO at 20 °C (33.55 μg/g). on average, CL adsorbed the most amount of CO at 110 °C.
The adsorbents in the wet and dried state and their adsorption capacities for different molecules (NO, SO 2 , CO) at different adsorbent temperatures (20 and 110 °C).
AC1-3, CL1-3, SiO 2 1-3—activated carbon, clay, and silicon dioxide, respectively (1-3—three granulometric fractions; see Table 1 ), CEL—cellulose, PP—polypropylene.
During the adsorption of NO on AC, there was a significant competitive effect between NO and SO 2 at both temperatures ( Figure 7 and Figure 8 ). As soon as most of the AC adsorption sites were occupied by SO 2 molecules and the adsorbed amount began to decrease ( t = 550–950 s at 20 °C, 40–70 s at 110 °C; Figure 7 ), SO 2 began to replace NO molecules, resulting in NO forced desorption. The more porous the AC (smaller granulometric fraction), the earlier the desorption effect occurs (represented by AC1, AC2, and AC3 borderlines in Figure 7 ).
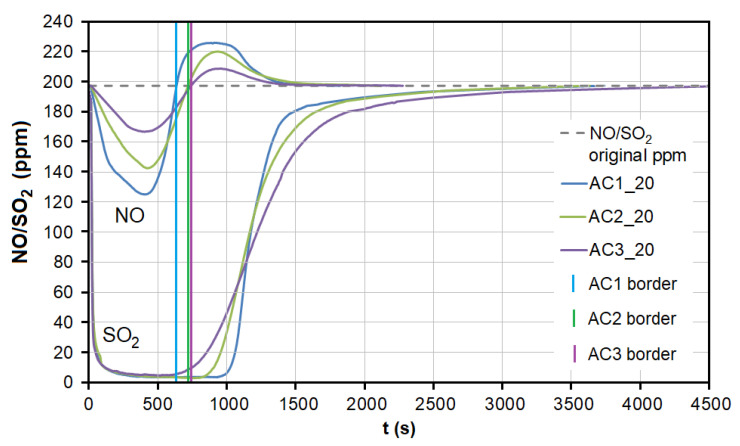
The dependence between SO 2 adsorption and NO desorption at the temperature of 20 °C. AC1, AC2, and AC3 border lines represent the beginning of the NO desorption effect for each adsorbent. AC1-3—activated carbon at different granulometric fractions (see Table 1 ).
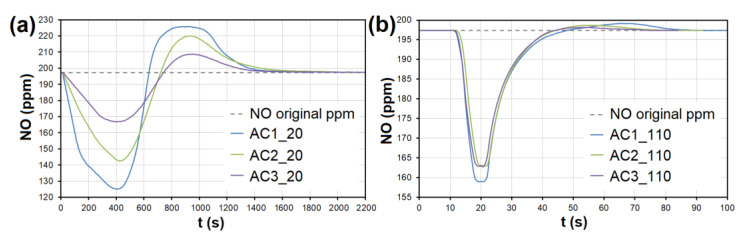
The adsorption curves showing NO adsorption on AC at adsorbent temperatures of ( a ) 20 °C and ( b ) 110 °C. The curves also show forced the desorption effect in the ranges of ( a ) 630–2150 s and ( b ) 43–93 s. AC1-3—activated carbon at different granulometric fractions (see Table 1 ).
At the temperature of 20 °C, a total of 214.32/92.66 μg/g of NO was adsorbed/desorbed on AC1, 163.92/60.86 μg/g on AC2, and 106.51/30.03 μg/g on AC3 ( Figure 8 ). This phenomenon was investigated in more detail using molecular dynamics (see Section 3.3 ). Forced desorption was not observed with any other adsorbent.
In the case of AC, the adsorption maximum of NO was reached gradually ( Figure 8 ), while the SO 2 adsorption maximum of SO 2 occurred immediately after the EG flow started ( Figure 9 ). The same was observed during SO 2 adsorption on CL, SiO 2 and CEL ( Figure 10 and Figure 11 ).
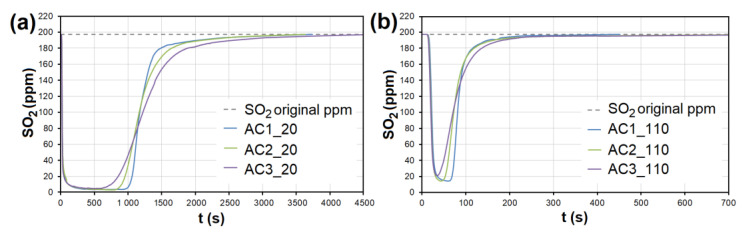
The adsorption curves showing SO 2 adsorption on AC at adsorbent temperatures of ( a ) 20 °C and ( b ) 110 °C. AC1-3—activated carbon at different granulometric fractions (see Table 1 ).
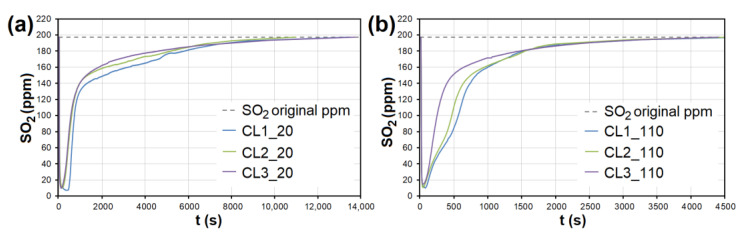
The adsorption curves showing SO 2 adsorption on CL at adsorbent temperatures of ( a ) 20 °C and ( b ) 110 °C. CL1-3—clay at different granulometric fractions (see Table 1 ).
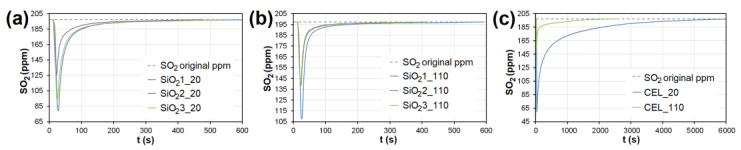
The adsorption curves showing SO 2 adsorption on SiO 2 at adsorbent temperatures of ( a ) 20°C and ( b ) 110 °C. ( c ) The adsorbing curve showing SO 2 adsorption on CEL at adsorbent temperatures of 20°C and 110 °C. SiO 2 1-3—silicon dioxide at different granulometric fractions (see Table 1 ), CEL—cellulose.
3.3. Molecular Modeling
CO 2 adsorption was not observed on any of the adsorbents, and CO was only adsorbed in negligible amounts and only at a temperature of 110 °C. Therefore, these two EG components were not molecularly modeled. Molecular dynamics clarified the competitive adsorption process of NO and SO 2 on the most adsorbing materials, i.e., AC (having the most specific adsorption process; Figure 12 ) and CL (MUS and NON). It showed NO forced desorption caused by SO 2 adsorption. Consequently, it was found with geometry optimization that SO 2 showed a lower E int with the surfaces than NO ( Figure 13 a,b), which correlated with the molecular dynamics results. The SO 2 molecules gradually occupied the top of the first surface, and the bottom of the second one. According to the notation “EG molecule_EG molecules number on the top of the surface/on the bottom of the surface_in the vacuum slab”, there were SO 2 _12/13_3 on AC, SO 2 _10/9_9 on MUS, and SO 2 _7/7_14 on NON ( Figure 12 ), while most NO molecules were expelled away from the surfaces (NO_3/3_22 for AC, NO_0/2_26 for MUS, and NO_1/1_26 for NON; Figure 12 ). The molecular dynamics results correspond to the experimentally determined sorption capacities, when higher sorption capacities were measured for SO 2 than for NO; and at 20 °C (293 K), AC showed higher sorption capacity than CL (MUS/NON; Table 3 ).
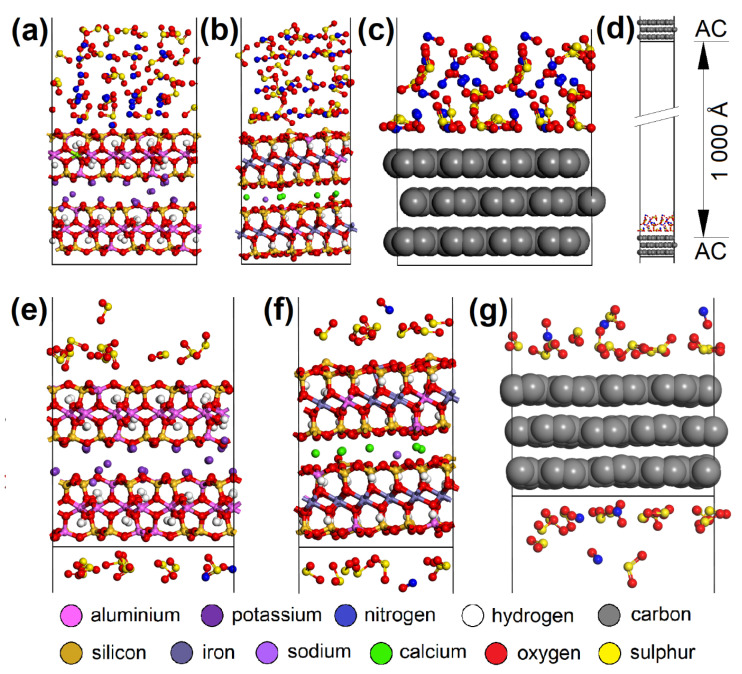
The initial molecular models containing 28 SO 2 and 28 NO molecules on the ( a ) MUS, ( b ) NON, and ( c ) AC surfaces. ( d ) The initial AC cell with EG molecules and the vacuum slab. The models after molecular dynamics showing the preference of SO 2 over NO molecules during the adsorption process on the ( e ) MUS, ( f ) NON, and ( g ) AC surfaces. MUS—muscovite, NON—nontronite, AC—activated carbon; EG—emission gas.
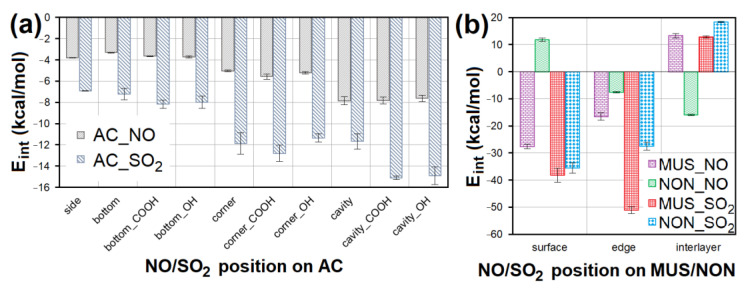
The E int comparison of models containing NO/SO 2 at different sites of ( a ) AC surface without or with -COOH/-OH groups and ( b ) MUS/NON surfaces. AC—activated carbon, MUS—muscovite, NON—nontronite, E int —interaction energy.
Due to the established correlation of molecular dynamics and geometry optimization results, the other models were only geometry optimized with one EG molecule on the surfaces in order to determine E int and observe molecular interactions in more detail.
Geometry optimization showed the suitability of the chemical composition of all sorbent materials for NO and SO 2 adsorption. An attractive interaction with NO or SO 2 was observed for all models containing AC. Average E int of models containing NO was 2× higher than models containing SO 2 on AC ( Figure 13 a). NO interacted most strongly with the cavities, cavity_COOH ( E int = −8.312 kcal/mol) and cavity_OH. The second strongest NO interactions were observed with the AC corner surfaces ( E int = −5.962 kcal/mol). In the case of SO 2 , the strongest interactions were observed for cavity_COOH ( E int = −15.360 kcal/mol) and cavity_OH. Corner_COOH ( E int = −13.877 kcal/mol) showed the second strongest interactions with SO 2 . These findings prove that the interaction of NO/SO 2 with AC is stronger, the more numerous surface atoms participating in the non-bonding interaction. The presence of the functional groups (mainly -COOH) is another important factor supporting the strength of AC interaction with NO/SO 2 via hydrogen bonds.
NO showed the strongest interaction with the MUS surface ( E int = −28.785 kcal/mol) and NON interlayer ( E int = −16.177 kcal/mol). The lowest E int showed the model containing SO 2 at the MUS edge ( E int = −52.357 kcal/mol); and the second strongest SO 2 interaction was with MUS and NON edge ( Figure 13 b). The MUS and NON interlayers in most cases showed a positive E int (repulsive force) with NO and SO 2 , due to a large number of cations in the interlayer of clay minerals causing a strong interaction between clay layers, and making it impossible for NO or SO 2 to penetrate. At the same time, these cations occupied all chemically suitable sites for interaction with NO and SO 2 . The average E int of models containing SO 2 on MUS and NON is significantly lower than in the case of models with NO.
All models containing NO placed at all sites of the CEL, PP and SiO 2 surfaces showed a weak negative interaction energy. The strongest interaction ( E int = −10.936 kcal/mol; Figure 14 a) was achieved in the case of NO lying in the corner of SiO 2 . The second lowest E int (−8.137 kcal/mol; Figure 14 a) was achieved for NO placed in the CEL cavity. SO 2 interacted with CEL, PP and SiO 2 surfaces several times stronger. The model with the strongest interaction of SO 2 in the CEL cavity showed 5× lower E int (−55.491 kcal/mol; Figure 14 b) than the model with the strongest interaction with NO. The second strongest interaction ( E int = −27.872 kcal/mol; Figure 14 b) was shown by SO 2 on the SiO 2 bottom. The average E int of models containing NO/SO 2 on PP was the highest.
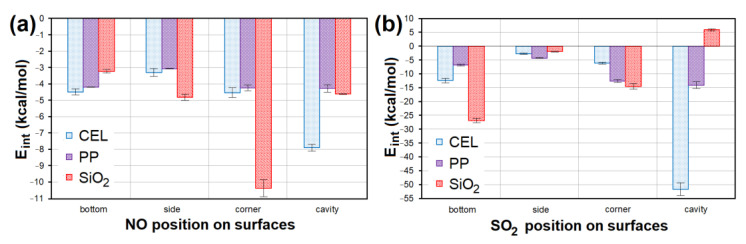
The E int comparison of models containing ( a ) NO and ( b ) SO 2 at different sites of CEL, PP and SiO 2 surfaces. CEL—cellulose, PP—polypropylene, SiO 2 —silicon dioxide, E int —interaction energy.
3.4. Adsorption Factors
The importance of chemical ( E int ) and textural ( S BET , S meso , V micro , V net ) properties on the adsorption capacity of adsorbents was determined ( Table 4 ). AC adsorption capacity was high for both NO and SO 2 , which was mainly correlated with the large surface area in spite of high E int values (i.e., weak interactions). The high CL adsorption capacity was due to a combination of both chemical and textural properties. A dominant factor of low SiO 2 and PP adsorption capacity was primarily textural properties (despite strong interactions). The high CEL adsorption capacity was mainly provided by the presence of functional groups strongly interacting with SO 2 molecules.
Determining the importance of E int or textural properties ( S BET , S meso , V micro , V net ) on adsorption capacity of the adsorbents having 0.16–0.315 mm granulometry.
* denotes dominant adsorption factor, i.e., chemical ( E int ) or textural ( S BET , S meso , V micro , V net ). AC—activated carbon, CL—clay, SiO 2 —silicon dioxide, CEL—cellulose, PP—polypropylene, S BET —specific surface area, S meso —mesopore–macropore surface area, V micro —micropore volume, V net —net pore volume, n. d.—not defined, E int —interaction energy.
Furthermore, agreement between the experiment and molecular modeling was generally more common for lower sorption capacities for NO (corresponding to higher E int values, i.e., weaker interaction) compared to higher SO 2 capacities (corresponding to lower E int values, i.e., stronger interaction).
In general, a significantly lower E int can be observed for models containing a molecule in a molecular pore (mainly CEL), which appears to be an ideal combination of chemical (more non-bonding interactions) and structural/steric (molecule size correlates with pore size) factors. The agreement of the force field-based molecular modeling results and experimental data demonstrate the modeling strategy used to be a suitable tool for analysis and prediction of interactions during the sorption process at the molecular level.
However, other modeling studies performed using more computationally demanding DFT methods have led to similar results for interactions between AC and NO/SO 2 [ 32 , 34 ]. Wang et al. [ 34 ] reported the same behavior of NO on AC—the strongest interactions were observed on AC basal plane (compare with “side” in Figure 13 a), weaker interactions with -OH group (compare with “bottom_OH” in Figure 13 a), and the weakest with -H (compare with “bottom” in Figure 13 a). Zhao et al. [ 32 ] found the same E int trend for SO 2 on AC, where the molecule interacted most strongly with -COOH groups (compare with “bottom_COOH” in Figure 13 a), more weakly with -OH (compare with “bottom_OH” in Figure 13 a), and the weakest with -H (compare with “bottom” in Figure 13 a).
The comparison demonstrates the usefulness of computationally less demanding force field-based molecular modeling in adsorption research. It also reveals that there is no need to take into account the partial amorphousness of AC, e.g., by a large amount of polyaromatic hydrocarbons [ 32 ]. Approximation of AC using graphite, which additionally enables the preparation of pores of the required size, is sufficient for the given purpose.
4. Conclusions
In this work, the competitive adsorption of emission gas, containing CO, NO, SO 2 and CO 2 , on adsorption materials with different chemical compositions was studied.
CO 2 was not adsorbed on any material. CO was adsorbed in small amounts and mainly at a temperature of 110 °C on AC, CL and CEL. At 20 °C, the AC showed the highest NO and SO 2 sorption capacity. At 110 °C, the highest NO and SO 2 sorption capacity was found for the CL.
The strongest interactions were exhibited between NO/SO 2 and corner or cavity surface types due to the larger number of interacting atoms. Carboxyl and hydroxyl functional groups strengthened the interaction of AC mainly with SO 2 , where hydrogen bonds were formed. NO and SO 2 showed attractive interactions with MUS and NON mainly on the surface and edge. Strong interactions between clay layers and interlayer cations prevented the penetration of NO and SO 2 into the interlayer space.
The dominant factor determining the sorption capacity of AC, SiO 2 and PP was textural (surface area, porosity)—while in the case of CEL, it was the chemical factors (chemical composition, functional groups). Both textural and chemical factors played an important role in adsorption on CL.
The effects of competitive adsorption of emission components on sorption capacity were determined and further clarified using force field-based molecular modeling. The modeling strategy made it possible to simulate surfaces with different and controllable structural character. Geometry optimization and molecular dynamics results agreed well with experimental data allowing the modeling strategy to be further used in studies of interactions during adsorption, or for a prediction of adsorption phenomena.
Author Contributions
Conceptualization, A.V.; methodology, A.V. and J.T.; software, A.V. and J.T.; validation, A.V. and T.N.; formal analysis, A.V., L.M. and K.M.K.; investigation, A.V. and T.N.; resources, J.K. and V.P.; data curation, A.V.; writing—original draft preparation, A.V.; writing—review and editing, J.T.; visualization, A.V.; supervision, J.T.; project administration, A.V.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Doctoral grant competition VSB—Technical University of Ostrava, reg. no. CZ.02.2.69/0.0/0.0/19_073/0016945 within the Operational Programme Research, Development and Education, under project DGS/TEAM/2020-005 “Low-potencial waste heat recovery”. Large Research Infrastructure ENREGAT supported by the Ministry of Education, Youth and Sports of the Czech Republic (project No. LM2018098) also contributed to the realization of this work.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
- 1. Brusseau M.L., Pepper I.L., Gerba C.P. Environmental and Pollution Science. 3rd ed. Elsevier; Amsterdam, The Netherlands: 2019. [ Google Scholar ]
- 2. Henzel C.K. Investigating Manufacturing Pollution. 1st ed. Child’s World; Parker, CO, USA: 2022. [ Google Scholar ]
- 3. Tillman D.A. Coal-Fired Electricity and Emissions Control: Efficiency and Effectiveness. 1st ed. Butterworth-Heinemann; Oxford, UK: 2018. [ Google Scholar ]
- 4. Matz C.J., Egyed M., Hocking R., Seenundun S., Charman N., Edmonds N. Human health effects of traffic-related air pollution (TRAP): A scoping review protocol. Syst. Rev. 2019;8:223. doi: 10.1186/s13643-019-1106-5. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. Boot-Handford M.E., Abanades J.C., Anthony E.J., Blunt M.J., Brandani S., Mac Dowell N., Fernández J.R., Ferrari M.C., Gross R., Hallett J.P., et al. Carbon capture and storage update. Energy Environ. Sci. 2014;7:130–189. doi: 10.1039/C3EE42350F. [ DOI ] [ Google Scholar ]
- 6. Werther J., Saenger M., Hartge E.U., Ogada T., Siagi Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000;26:1–27. doi: 10.1016/S0360-1285(99)00005-2. [ DOI ] [ Google Scholar ]
- 7. Obey G., Adelaide M., Ramaraj R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022;7:109–115. doi: 10.1016/j.jobab.2021.12.001. [ DOI ] [ Google Scholar ]
- 8. Zheng Q., Li Z., Watanabe M. Production of solid fuels by hydrothermal treatment of wastes of biomass, plastic, and biomass/plastic mixtures: A review. J. Bioresour. Bioprod. 2022;7:221–244. doi: 10.1016/j.jobab.2022.09.004. [ DOI ] [ Google Scholar ]
- 9. Bai J., Yu C., Li L., Wu P., Luo Z., Ni M. Experimental study on the NO and N2O formation characteristics during biomass combustion. Energy Fuels. 2013;27:515–522. doi: 10.1021/ef301383g. [ DOI ] [ Google Scholar ]
- 10. Jones J.M., Lea-Langton A.R., Ma L., Pourkashanian M., Williams A. Pollutants Generated by the Combustion of Solid Biomass Fuels. 1st ed. Springer; London, UK: 2014. The Combustion of Solid Biomass; pp. 25–43. [ Google Scholar ]
- 11. Yu C.H., Huang C.H., Tan C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012;12:745–769. doi: 10.4209/aaqr.2012.05.0132. [ DOI ] [ Google Scholar ]
- 12. Sircar S. Basic research needs for design of adsorptive gas separation processes. Ind. Eng. Chem. Res. 2006;45:5435–5448. doi: 10.1021/ie051056a. [ DOI ] [ Google Scholar ]
- 13. Zhu B., Liu Q., Zhou Q., Yang J., Ding J., Wen J. Absorption of carbon dioxide from flue gas using blended amine solutions. Chem. Eng. Technol. 2014;37:635–642. doi: 10.1002/ceat.201300240. [ DOI ] [ Google Scholar ]
- 14. Choi S., Drese J.H., Jones C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem. 2009;2:796–854. doi: 10.1002/cssc.200900036. [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Samanta A., Zhao A., Shimizu G.K., Sarkar P., Gupta R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012;51:1438–1463. doi: 10.1021/ie200686q. [ DOI ] [ Google Scholar ]
- 16. Wang Y., Zhou Y., Liu C., Zhou L. Comparative studies of CO2 and CH4 sorption on activated carbon in presence of water. Colloids Surfaces A Physicochem. Eng. Asp. 2008;322:14–18. doi: 10.1016/j.colsurfa.2008.02.014. [ DOI ] [ Google Scholar ]
- 17. Zhang J., Liu K., Clennell M.B., Dewhurst D.N., Pan Z., Pervukhina M., Han T. Molecular simulation studies of hydrocarbon and carbon dioxide adsorption on coal. Pet. Sci. 2015;12:692–704. doi: 10.1007/s12182-015-0052-7. [ DOI ] [ Google Scholar ]
- 18. Bezerra D.P., Oliveira R.S., Vieira R.S., Cavalcante C.L., Azevedo D. Adsorption of CO2 on nitrogen-enriched activated carbon and zeolite 13X. Adsorption. 2011;17:235–246. doi: 10.1007/s10450-011-9320-z. [ DOI ] [ Google Scholar ]
- 19. Li Z.S., Cai N.S., Huang Y.Y., Han H.J. Synthesis, experimental studies, and analysis of a new calcium-based carbon dioxide absorbent. Energy Fuels. 2005;19:1447–1452. doi: 10.1021/ef0496799. [ DOI ] [ Google Scholar ]
- 20. Franchi R.S., Harlick P.J., Sayari A. Applications of pore-expanded mesoporous silica. 2. Development of a high-capacity, water-tolerant adsorbent for CO2. Ind. Eng. Chem. Res. 2005;44:8007–8013. doi: 10.1021/ie0504194. [ DOI ] [ Google Scholar ]
- 21. Ding Y., Alpay E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 2000;55:3461–3474. doi: 10.1016/S0009-2509(99)00596-5. [ DOI ] [ Google Scholar ]
- 22. Srivatsa S.C., Bhattacharya S. Amine-based CO2 capture sorbents: A potential CO2 hydrogenation catalyst. J. CO2 Util. 2018;26:397–407. doi: 10.1016/j.jcou.2018.05.028. [ DOI ] [ Google Scholar ]
- 23. Li J.R., Kuppler R.J., Zhou H.C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009;38:1477–1504. doi: 10.1039/b802426j. [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. Wang H., Bai J.Q., Yin Y., Wang S.F. Experimental and numerical study of SO2 removal from a CO2/SO2 gas mixture in a Cu-BTC metal organic framework. J. Mol. Graph. Model. 2020;96:107533. doi: 10.1016/j.jmgm.2020.107533. [ DOI ] [ PubMed ] [ Google Scholar ]
- 25. Ozensoy E., Goodman D.W. Vibrational spectroscopic studies on CO adsorption, NO adsorption CO+ NO reaction on Pd model catalysts. Phys. Chem. Chem. Phys. 2004;6:3765–3778. doi: 10.1039/b402302a. [ DOI ] [ Google Scholar ]
- 26. Joshi A.M., Tucker M.H., Delgass W.N., Thomson K.T. CO adsorption on pure and binary-alloy gold clusters: A quantum chemical study. J. Chem. Phys. 2006;125:194707. doi: 10.1063/1.2375094. [ DOI ] [ PubMed ] [ Google Scholar ]
- 27. Abdulrasheed A.A., Jalil A.A., Triwahyono S., Zaini M.A.A., Gambo Y., Ibrahim M. Surface modification of activated carbon for adsorption of SO2 and NOX: A review of existing and emerging technologies. Renew. Sustain. Energ. Rev. 2018;94:1067–1085. doi: 10.1016/j.rser.2018.07.011. [ DOI ] [ Google Scholar ]
- 28. Niu J., Miao J., Zhang H., Guo Y., Li L., Cheng F. Focusing on the impact of inherent minerals in coal on activated carbon production and its performance: The role of trace sodium on SO2 and/or NO removal. Energy. 2023;263:125638. doi: 10.1016/j.energy.2022.125638. [ DOI ] [ Google Scholar ]
- 29. Rodriguez J.A. The chemical properties of bimetallic surfaces: Importance of ensemble and electronic effects in the adsorption of sulfur and SO2. Prog. Surf. Sci. 2006;81:141–189. doi: 10.1016/j.progsurf.2006.02.001. [ DOI ] [ Google Scholar ]
- 30. Yi H., Deng H., Tang X., Yu Q., Zhou X., Liu H. Adsorption equilibrium and kinetics for SO2, NO, CO2 on zeolites FAU and LTA. J. Hazard. Mater. 2012;203:111–117. doi: 10.1016/j.jhazmat.2011.11.091. [ DOI ] [ PubMed ] [ Google Scholar ]
- 31. Wang L., Xuan C., Zhang X., Sun R., Cheng X., Wang Z., Ma C. NOx Adsorption Mechanism of Coal-Based Activated Carbon Modified with Trace Potassium: In Situ DRIFTS and DFT Study. Energy Fuels. 2022;36:7633–7650. doi: 10.1021/acs.energyfuels.2c00814. [ DOI ] [ Google Scholar ]
- 32. Zhao R., Liu G., Wei G., Gao J., Lu H. Analysis of SO2 Physisorption by Edge-Functionalized Nanoporous Carbons Using Grand Canonical Monte Carlo Methods and Density Functional Theory: Implications for SO2 Removal. ACS Omega. 2021;6:33735–33746. doi: 10.1021/acsomega.1c05000. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Zeng W., Tan S.J., Liu M., Zhang D., Liu L., Do D.D. New Insights into the Capture of Low-level Gaseous Pollutants in Indoor Environment by Carbonaceous Materials: Effects of Functional Groups, Pore Size, and Presence of Moist. Sep. Purif. Technol. 2022;298:121652. doi: 10.1016/j.seppur.2022.121652. [ DOI ] [ Google Scholar ]
- 34. Wang J., Yang M., Deng D., Qiu S. The adsorption of NO, NH3, N2 on carbon surface: A density functional theory study. J. Mol. Model. 2017;23:262. doi: 10.1007/s00894-017-3429-2. [ DOI ] [ PubMed ] [ Google Scholar ]
- 35. Najser T., Gaze B., Knutel B., Verner A., Najser J., Mikeska M., Chojnacki J., Němček O. Analysis of the Effect of Catalytic Additives in the Agricultural Waste Combustion Process. Materials. 2022;15:3526. doi: 10.3390/ma15103526. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 36. Towler G., Sinnott R. Chemical Engineering Design: Principles, Practice and Economic of Plant and Process Design. 2nd ed. Elsevier; Amsterdam, The Netherlands: 2013. Process Flowsheet Development; pp. 33–102. [ Google Scholar ]
- 37. Das T.K. Industrial Environmental Management: Engineering, Science, and Policy. 1st ed. Wiley; Hoboken, NJ, USA: 2020. Industrial Pollution Sources, Its Characterization, Estimation, and Treatment; pp. 71–113. [ Google Scholar ]
- 38. BIOVIA . Materials Studio 7.0. Dassault Systèmes; San Diego, CA, USA: 2013. [ Google Scholar ]
- 39. Chen P., Nishiyama Y., Putaux J.L., Mazeau K. Diversity of potential hydrogen bonds in cellulose I revealed by molecular dynamics simulation. Cellulose. 2014;21:897–908. doi: 10.1007/s10570-013-0053-x. [ DOI ] [ Google Scholar ]
- 40. Verenich S., Paul S., Pourdeyhimi B. Surface and bulk properties of glycidyl methacrylate modified polypropylene: Experimental and molecular modeling studies. J. Appl. Polym. Sci. 2008;108:2983–2987. doi: 10.1002/app.27780. [ DOI ] [ Google Scholar ]
- 41. Wyckoff R.W.G. Crystal Structures. 2nd ed. Wiley; New York, NY, USA: 1963. [ Google Scholar ]
- 42. Weiss Z., Kužvart M. Clay Minerals: Their Nanostructure and Utilization. 1st ed. Karolinum; Prague, Czech Republic: 2005. [ Google Scholar ]
- 43. Bednárek J., Matějová L., Jankovská Z., Vaštyl M., Sokolová B., Peikertová P., Šiler P., Verner A., Tokarský J., Koutník I., et al. The Influence of Structural Properties on the Adsorption Capacities of Microwave-assisted Biochars for Metazachlor Removal from Aqueous Solutions. J. Environ. Chem. Eng. 2022;10:108003. doi: 10.1016/j.jece.2022.108003. [ DOI ] [ Google Scholar ]
- 44. Sun H. COMPASS: An ab initio force-field optimized for condensed-phase applications—Overview with details on alkane and benzene compounds. J. Phys. Chem. 1998;102:7338–7364. doi: 10.1021/jp980939v. [ DOI ] [ Google Scholar ]
- 45. Bazooyar F., Momany F.A., Bolton K. Validating empirical force fields for molecular-level simulation of cellulose dissolution. Comput. Theor. Chem. 2012;984:119–127. doi: 10.1016/j.comptc.2012.01.020. [ DOI ] [ Google Scholar ]
- 46. Deckers F., Rasim K., Schröder C. Molecular dynamics simulation of polypropylene: Diffusion and sorption of H2O, H2O2, H2, O2 and determination of the glass transition temperature. J. Polym. Res. 2022;29:463. doi: 10.1007/s10965-022-03304-y. [ DOI ] [ Google Scholar ]
- 47. Vijayakumar S.D., Ridzuan N. Molecular interaction study on Gemini surfactant and nanoparticles in wax inhibition of Malaysian crude oil. Asia-Pac. J. Chem. Eng. 2021;16:e2700. doi: 10.1002/apj.2700. [ DOI ] [ Google Scholar ]
- 48. Rai B., Sathish P., Tanwar J., Moon K.S., Fuerstenau D.W. A molecular dynamics study of the interaction of oleate and dodecylammonium chloride surfactants with complex aluminosilicate minerals. J. Colloid Interface Sci. 2011;362:510–516. doi: 10.1016/j.jcis.2011.06.069. [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Thommes M., Kaneko K., Neimark A.V., Olivier J.P., Rodriguez-Reinoso F., Rouquerol J., Sing K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report) Pure Appl. Chem. 2015;87:1051–1069. doi: 10.1515/pac-2014-1117. [ DOI ] [ Google Scholar ]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
- View on publisher site
- PDF (6.9 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES
VIDEO
COMMENTS
This value was crucial for the dosages chosen in competitive adsorption experiments. Here a total dosage of 4 mg/g cem was applied to ensure that all kinetic adsorption and desorption processes occur within the plateau region. 5.2.2. UV-vis-absorption spectroscopy.
It should be reiterated that multi-component breakthrough experiments can offer insight into competitive adsorption equilibrium and provide validation to the process model used in simulation studies. A multi-component adsorption breakthrough experiment is performed the same way that a single-component experiment is performed.
The pressure range of the competitive adsorption experiment for multi-component gas was 0.1-1.0 MPa, and 8 equilibrium pressure adsorption points were selected. The equilibrium time of each adsorption point exceeded 12 h, and the experiments were conducted under constant temperature conditions of 20, 30 and 40 °C, respectively. ...
Competitive Adsorption/Desorption Experiments. Competitive adsorption/desorption kinetic experiments exhibited similar observation patterns for Pb 2+ or Co 2+ competitive adsorption with Mg 2+ (Figure 4 and Figure 5; note that the y-axes have different scales on these two figures).
The binary equilibrium adsorption experiments of As(V) and F - were investigated using activated carbon as an adsorbent, and the competitive adsorption behavior modeled using JAMM isotherm. The binary batch experiments were conducted at room temperature of 25 °C in an incubator shaker for 150 rpm placed for 48 h for equilibrium.
Herein, goethite primarily exposing the {1 1 0} facet, was employed as the model iron mineral to investigate the competitive adsorption behavior of As(V), Sb(V), and P(V) via batch experiments leveraging EXAFS, CD-MUSIC model, and DFT calculations. Results showed that observed co-adsorption behaviors were attributed to the comprehensive action ...
In a multicomponent systems, the adsorption of Pb 2+, Cu 2+, and Ni 2+ by date seed biochar exhibited competitive behavior. Compared to single component systems, the adsorption capacities of each ion were reduced by 48-75% in both batch and column experiments.
The solubility and bioavailability of arsenic in the environment are to a large extent governed by adsorption reactions with iron (hydr)oxides, the extent of which is affected by competitive interactions with other ions, for example, phosphate. Here, batch experiments were performed with ferrihydrite suspensions to determine the adsorption of arsenate [As(V)] and phosphate (PO4) at different ...
This work is focused on competitive adsorption experiments and force field-based molecular modeling of the interactions at the molecular level. Emission gas, containing CO, NO, SO 2, and CO 2, was adsorbed on activated carbon, clay mineral, silicon dioxide, cellulose, or polypropylene at two different temperatures.
Interestingly, the effect of competitive adsorption was less pronounced in Phase 3 of this experiment when the more complete colloid suspension acted to facilitate metal mobility, in particular for Zn, when the input concentration was reached immediately at the start of Phase 3, regardless of metal solution composition.